NMR Discussion
These NMR spectra are scaled to 80mm height.
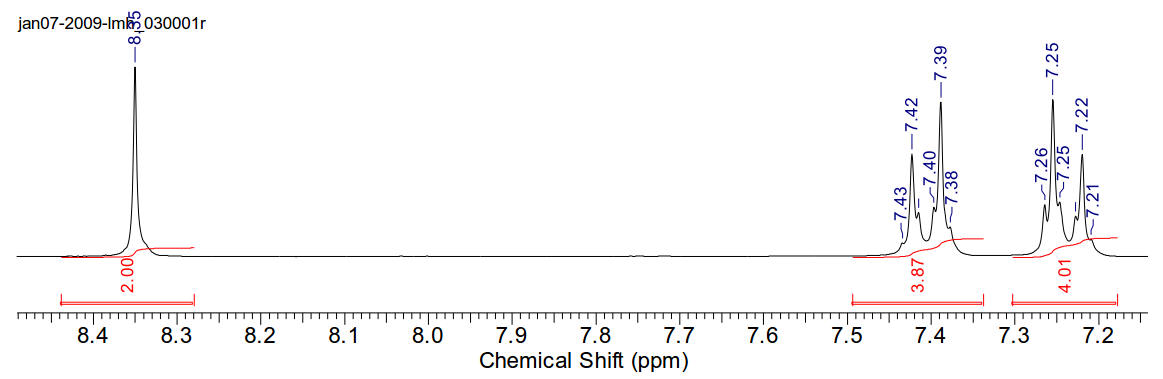
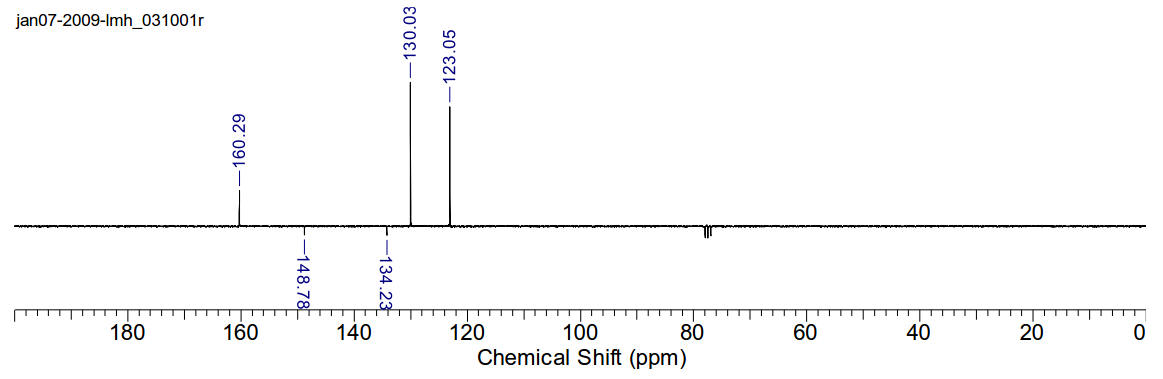
1,4-di(4-chlorophenyl)-1,4-diazabutadiene
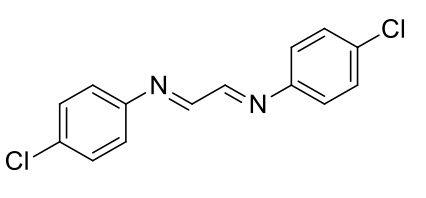
C14H10Cl2N2
277.15gmol-1
1H-NMR (400MHz, CDCl3) δ 8.35 (s, 2H), 7.41 (dt, J=2Hz,
J=9Hz, 4H), 7.24 (dt, J=8.75Hz, J=2.2Hz).
13C-NMR (400MHz, CDCl3) δ 160.29, 148.78, 134.23, 130.03,
123.05.
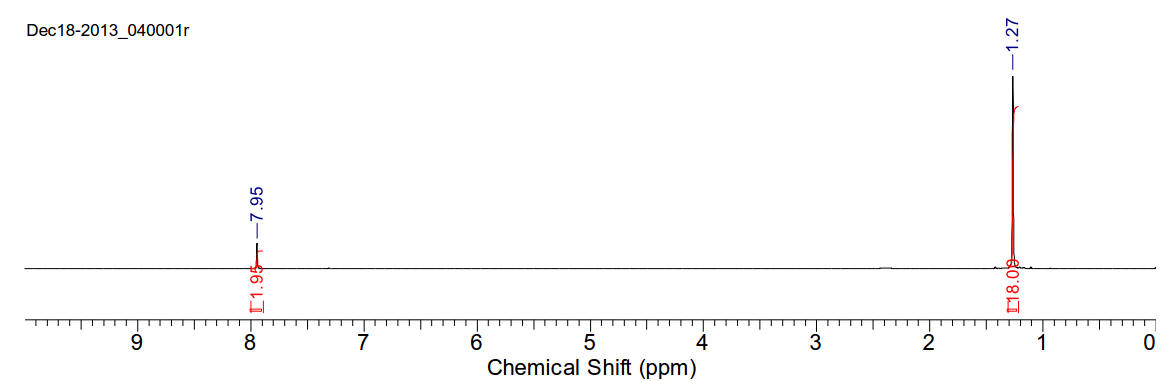
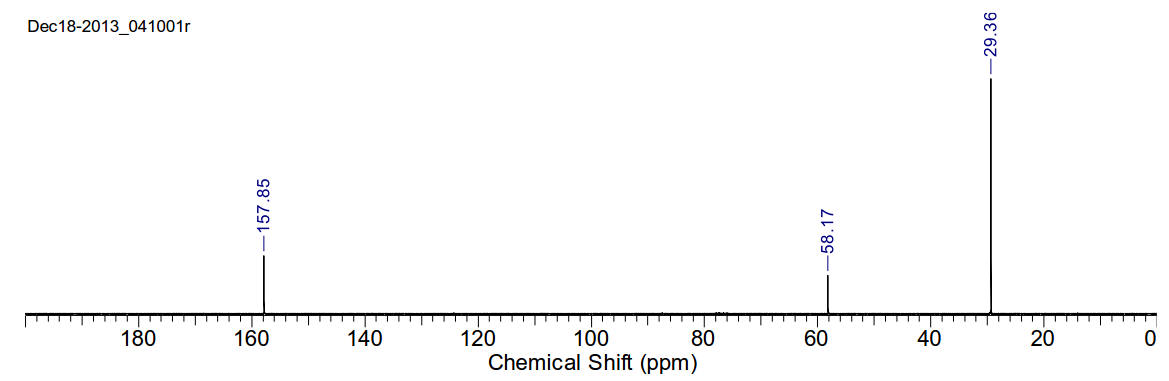
1,4-di(tert-butyl)-1,4-diazabutadiene
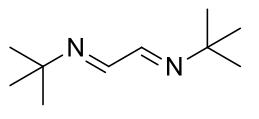
C10H20N2 168.28gmol-1
1H-NMR (400MHz, CDCl3) δ 7.95 (s, 2H), 1.27 (s, 18H).
1H-NMR (400MHz, D2O) δ 4.69 (s, 18H),
13C-NMR (400MHz, CDCl3) δ 157.85, 58.17, 29.36.
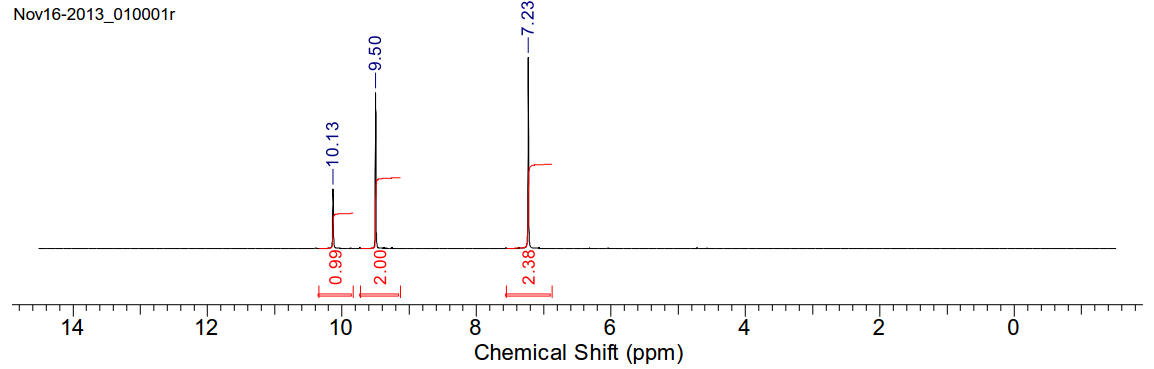
Imidazole
C3H4N2 68.08gmol-1
White crystals soluble with water and moderately soluble in chloroform. The
4(5)- protons are equivalent.
1H-NMR (400MHz, CDCl3) δ 12.42 (s, 1H), 7.73 (s, 1H), 7.13
(s, 1H)
1H-NMR (400MHz, D2O) δ 10.13 (s, 1H), 9.50 (s, 2H), 7.23
(s, 2H)
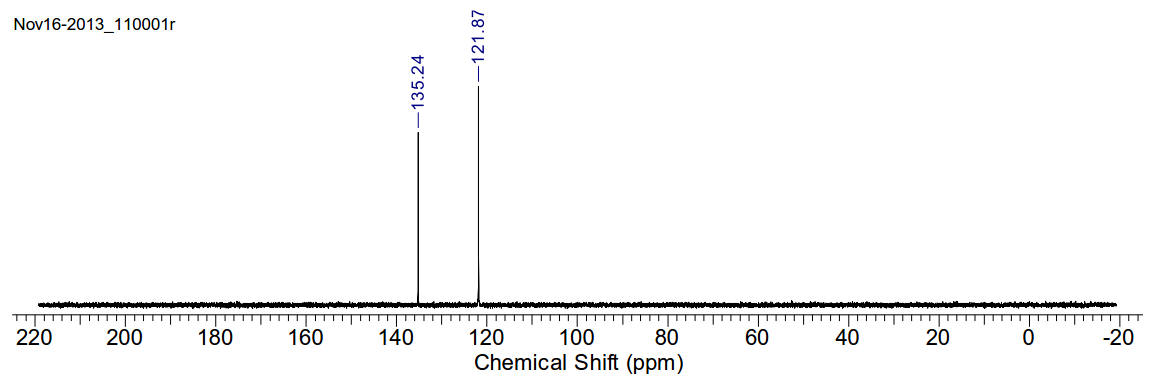
13C-NMR (400MHz, CDCl3) δ 135.24, 121.87
1-methylimidazole
C4H6N2 82.10gmol-1
A slight tan viscous liquid miscible with both water and chloroform.
1H-NMR (400MHz, CDCl3) δ 7.42 (s, 1H), 7.04 (s, 1H), 6.88
(s, 1H), 3.66 (s, 3H)
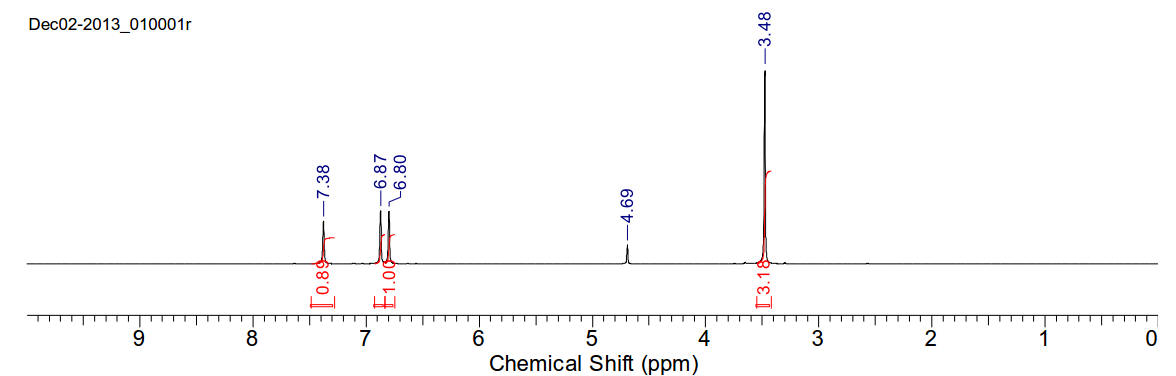
1H-NMR (400MHz, D2O) δ 7.38 (s, 1H), 6.87 (s, 1H), 6.80
(s, 1H), 3.48 (s, 3H)
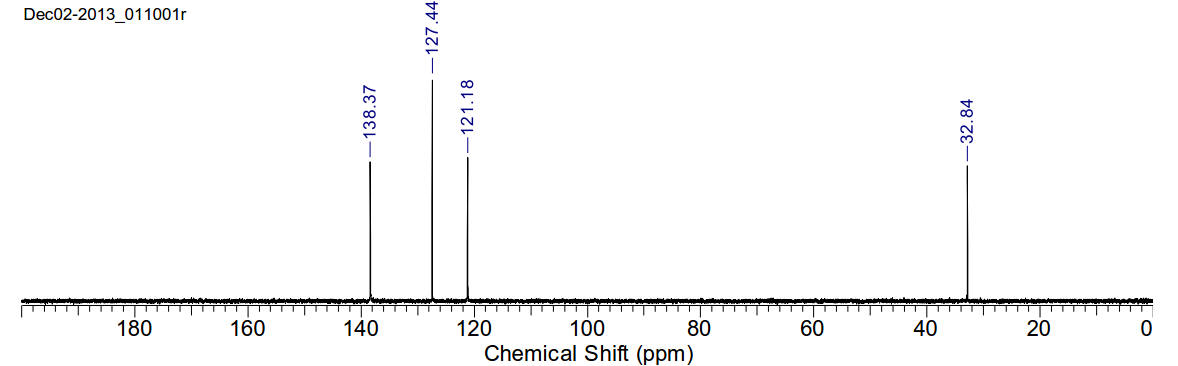
13C-NMR (400MHz, D2O) δ 138.37, 127.44, 121.18, 32.84
1H-NMR (400MHz, MeOD) δ 7.6 (s, 1H), 7.08 (s, 1H), 6.97 (s, 1H), 3.74
(3H)
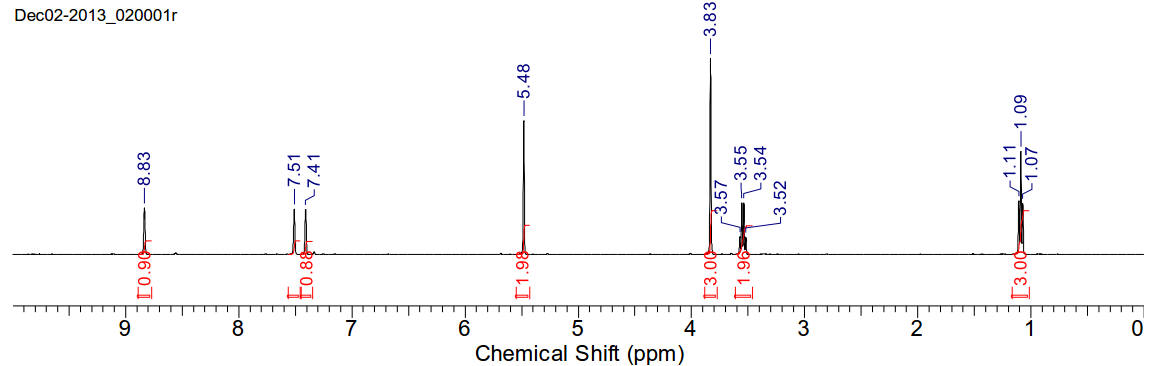
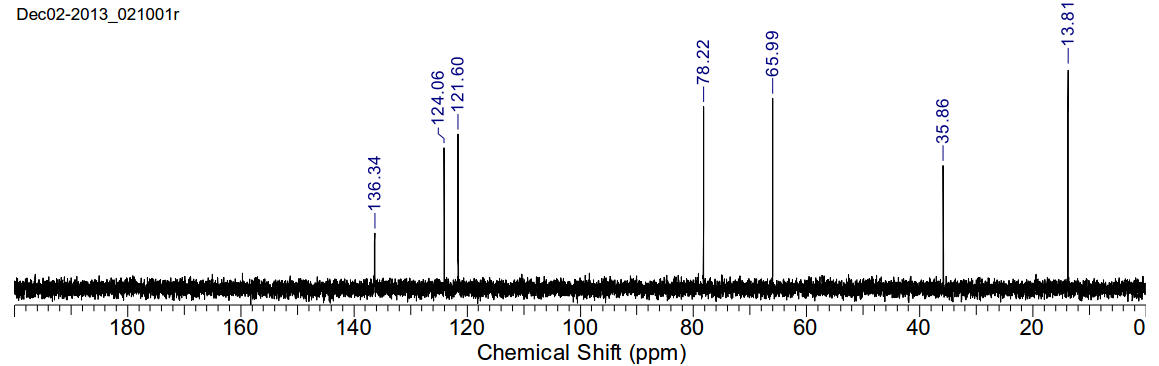
1-methyl-3-ethoxymethyl-imidazolium chloride
C7H13N2OCl 176.64gmol-1
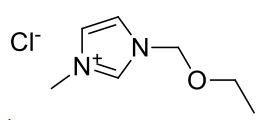
1H-NMR (400MHz, D2O) δ 8.83 (s, 1H), 7.51 (s, 1H), 7.41
(s, 1H), 5.48 (s, 2H), 3.83 (s, 3H), 3.545 (q, J=7.04Hz, 2H), 1.09 (t, J=7.04Hz,
3H)
13C-NMR (400MHz, D2O) δ 136.34, 124.06, 121.60, 78.22,
65.99, 35.86, 13.81
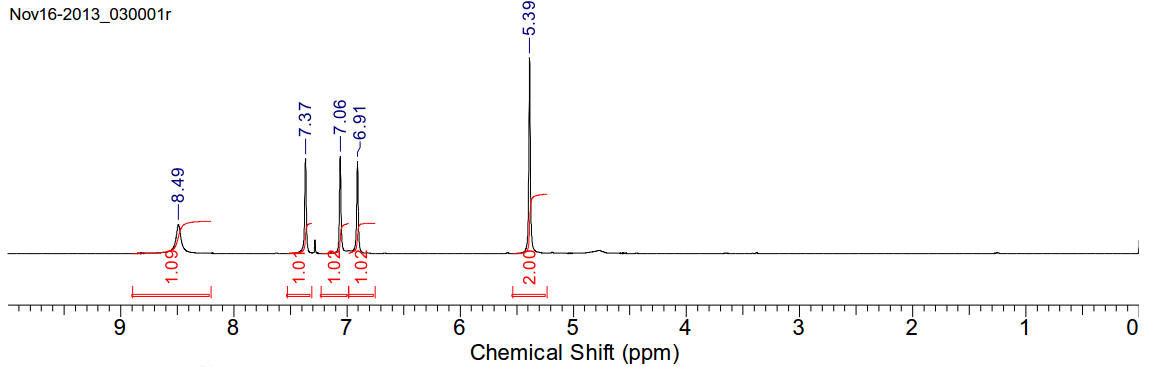
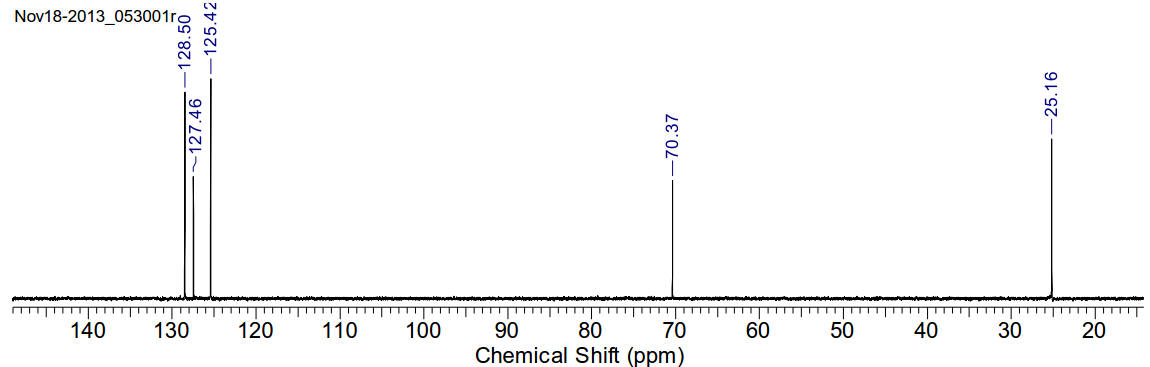
1-hydroxymethylimidazole
C4H6N2O 98.10gmol-1
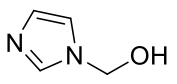
A waxy solid soluble in chloroform, acetonitrile and dichloromethane. * Reacts
with water.
1H-NMR (400MHz, CDCl3) δ 8.49 (s, 1H), 7.37 (s, 1H), 7.06
(s, 1H), 6.91 (s, 1H),
5.39 (s, 2H)
13C-NMR (400MHz, CDCl3) δ 128.50, 127.46, 125.42, 70.37,
25.16
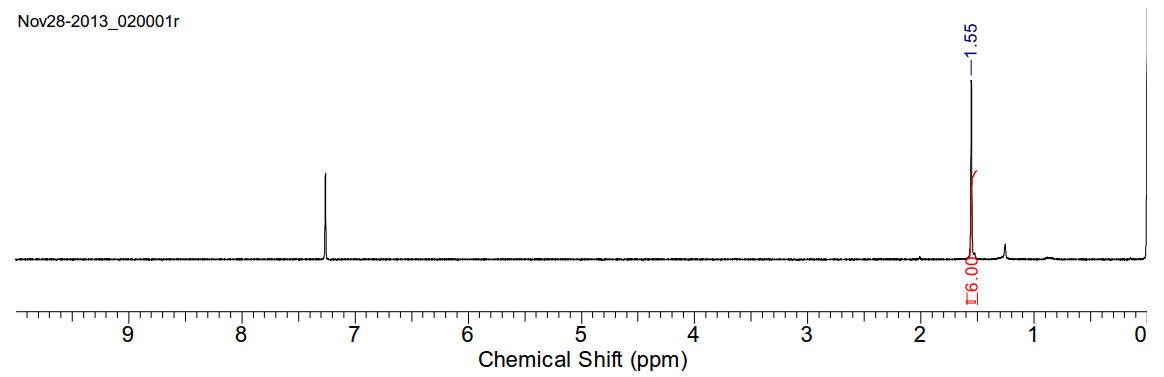
Glyoxal trimer dehydrate
C6H10O8 (210.14gmol-1)

1H-NMR (400MHz, CDCl3) δ 1.55 (s, 6H)
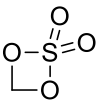
Methylene sulphate*
(CH2O4S)n 110.09gmol-1
*may be a dimer or polymer
1-chloromethylimidazole
C4H5N2Cl 116.55gmol-1
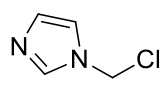
1H-NMR (400MHz, CDCl3) δ 7.45 (s,
1H), 7.39 (s, 1H), 7.31 (s, 1H), 6.10 (s, 2H)
Bis(imidazolyl)methane
C7H8N4 148.17gmol-1
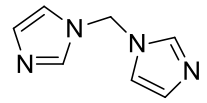
White crystal plates poorly soluble in water and chloroform.
1H-NMR (400MHz, D2O) δ 7.75 (s, 2H), 7.09 (s, 2H), 6.87
(s, 2H), 6.06 (s, 2H)
1H-NMR (400MHz, CDCl3) δ 7.66 (s, 2H), 7.12 (s, 2H), 7.00
(s, 2H), 6.00 (s, 2H)
13C-NMR (400MHz, D2O) δ 137.64, 128.77, 119.28, 55.96
13C-NMR (400MHz, CDCl3) δ 136.60, 131.18, 118.10, 56.33
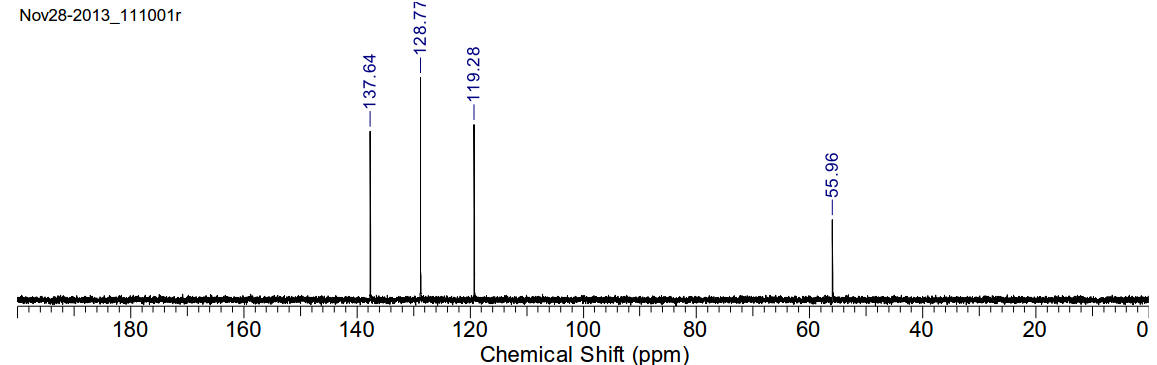
Bis(1-methylimidazolium-3-yl)methane chloride
C9H14Cl2N4
249.14gmol-1
1H-NMR (400MHz, D2O) δ 7.64 (s, 2H), 7.47 (s, 2H), 6.58
(s, 2H), 3.84 (s, 6H)
13C-NMR (400MHz, D2O) δ
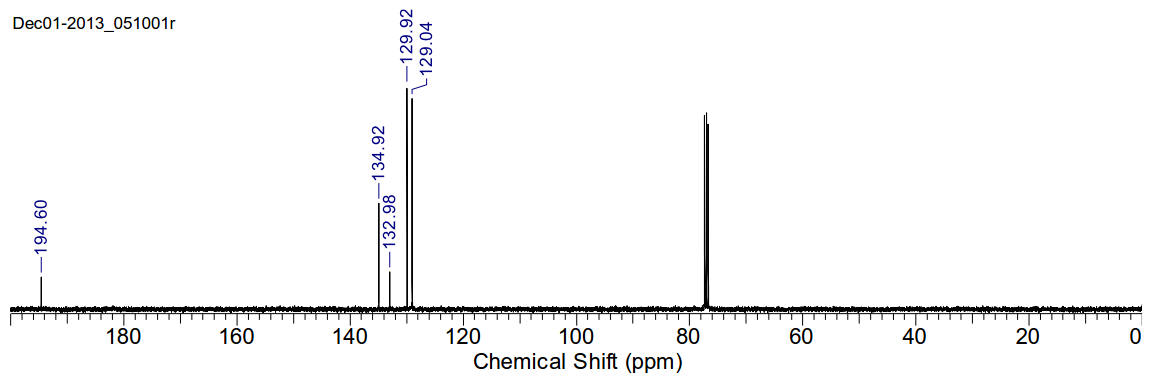
Benzil
C14H10O2 210.23gmol-1
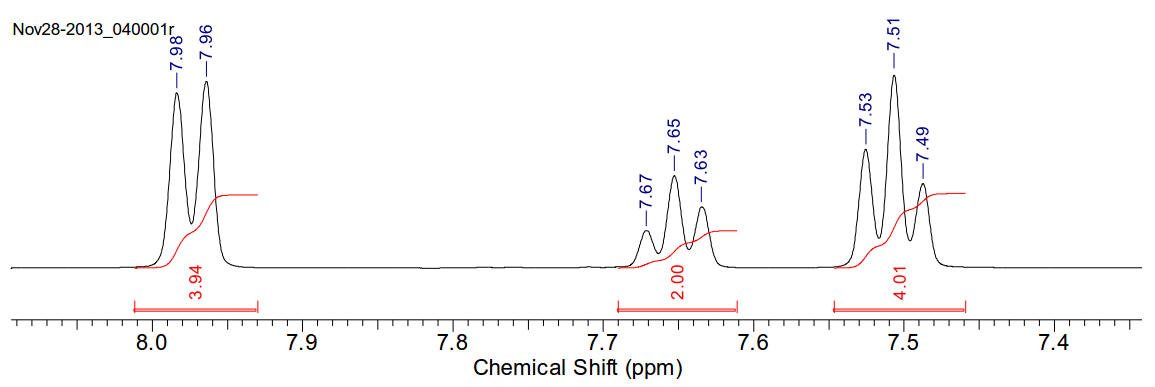
1H-NMR (400MHz, CDCl3) δ 7.97 (t,
J=7.92Hz, 4H), 7.65 (t, J=7.34Hz, 2H), 7.51 (t, J=7.58Hz, 4H)
13C-NMR (400MHz, CDCl3) δ 194.60,
134.92, 132.98, 129.92, 129.04
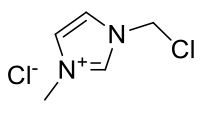
1-methyl-3-chloromethylimidazolium chloride
C5H8Cl2N2
167.04gmol-1
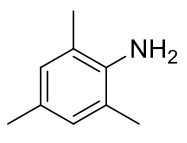
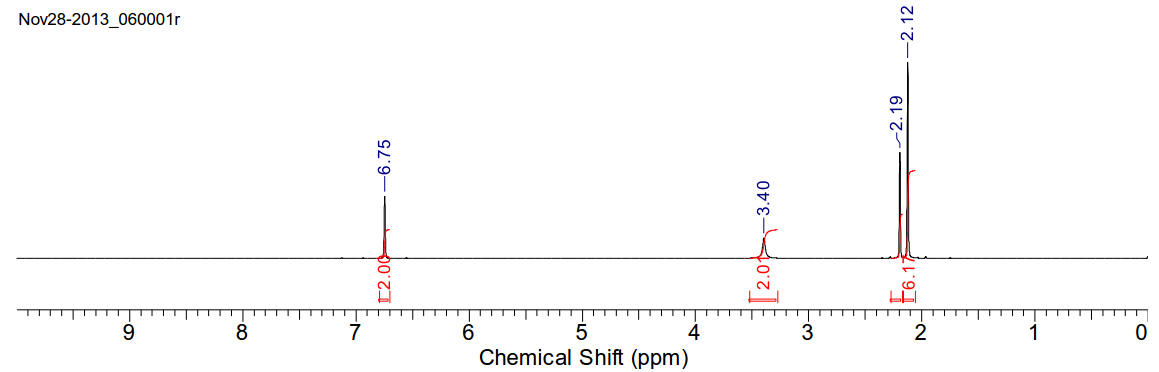
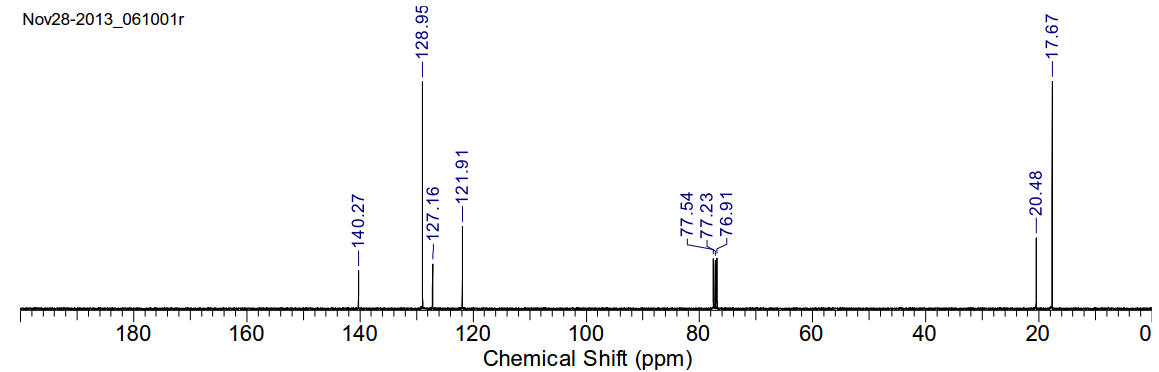
2,4,6-trimethylaniline (mesidine)
C9H13N 135.21gmol-1
A slightly red viscous oil. Miscible with chloroform.
1H-NMR (400MHz, CDCl3)
δ 6.75 (s, 2H), 3.40 (s, 2H), 2.19 (s, 3H), 2.13 (s, 6H)
1H-NMR (400MHz, D2O) δ 7.13 (t,
J=7.5Hz, 2H), 6.80-6.70 (m, 3H), 4.69 (s, 2H)
13C-NMR (400MHz, CDCl3) δ 140.27,
128.95, 127.16, 121.91, 20.48, 17.67
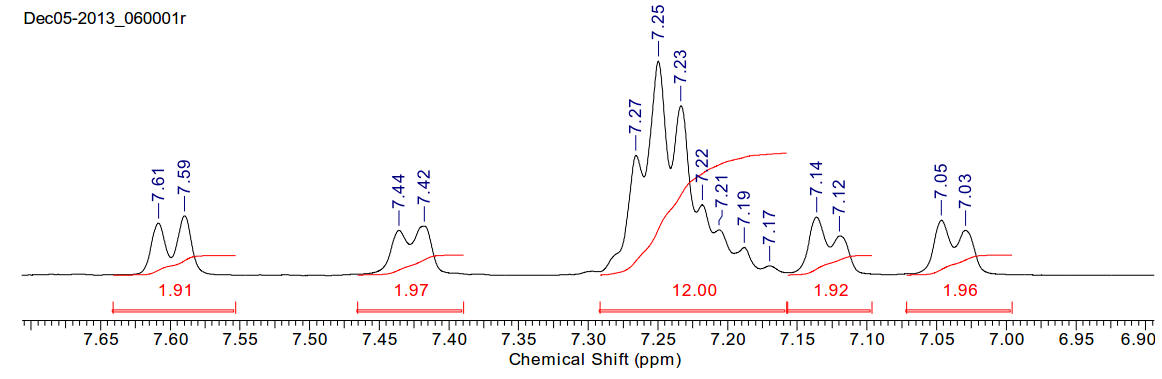
1,2,4,5-tetraphenyl imidazole
C27H20N2 372.46gmol-1
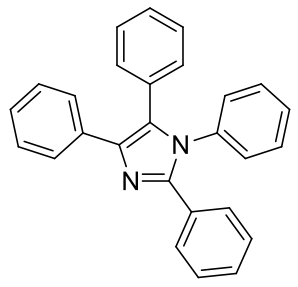
Insoluble in water, readily soluble in chloroform and
acetone.
1H-NMR (400MHz, CDCl3) δ 7.60 (d,
J=7.53Hz, 2H), 7.43 (d, J=7.24Hz, 2H), 7.30-7.15 (m, 12H), 7.13 (d, J=6.65Hz,
2H), 7.04 (d, J=6.94Hz, 2H)
13C-NMR (400MHz, CDCl3) δ
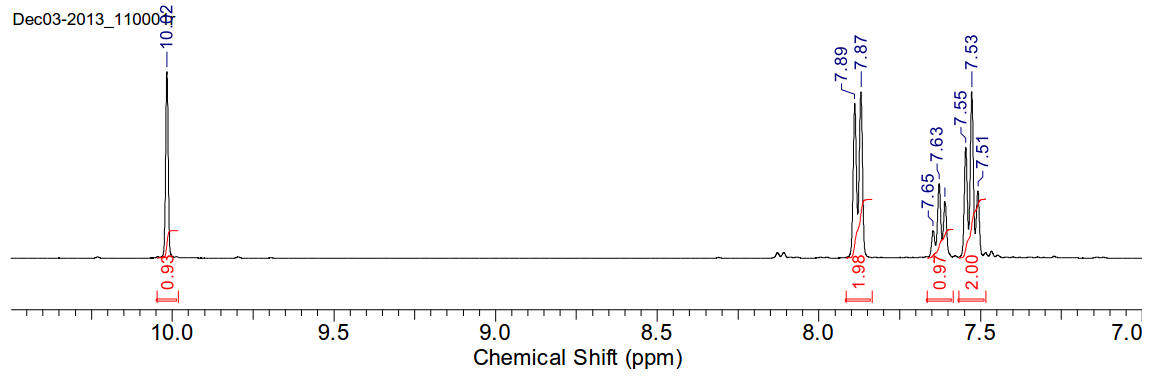
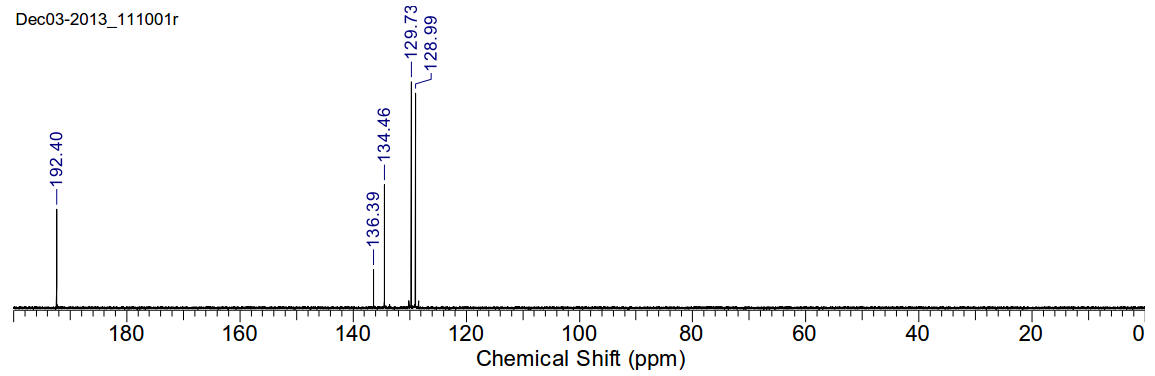
Benzaldehyde
C7H6O 106.12gmol-1
1H-NMR (400MHz, CDCl3) δ 10.02 (s,
1H), 7.88 (d, J=7.92Hz, 2H), 7.63 (t, J=7.3Hz, 1H), 7.53 (t, J=7.53Hz, 2H)
13C-NMR (400MHz, CDCl3) δ 192.40,
136.39, 134.46, 129.73, 128.99
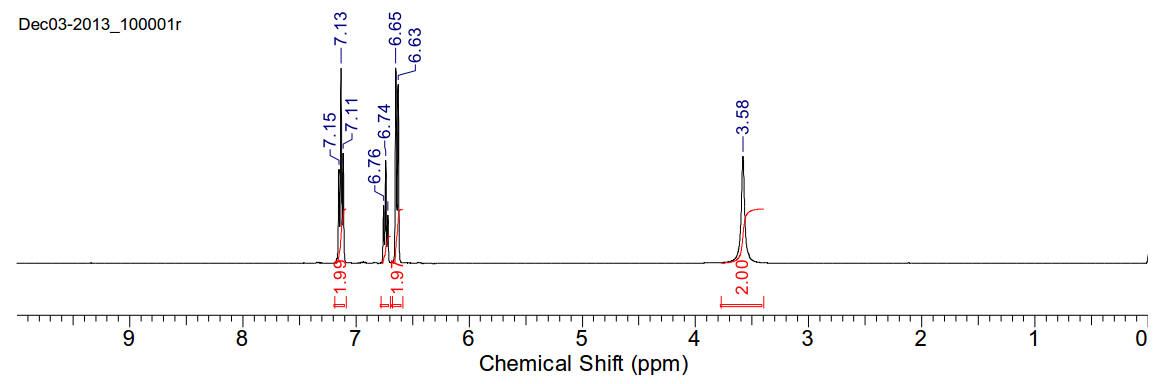
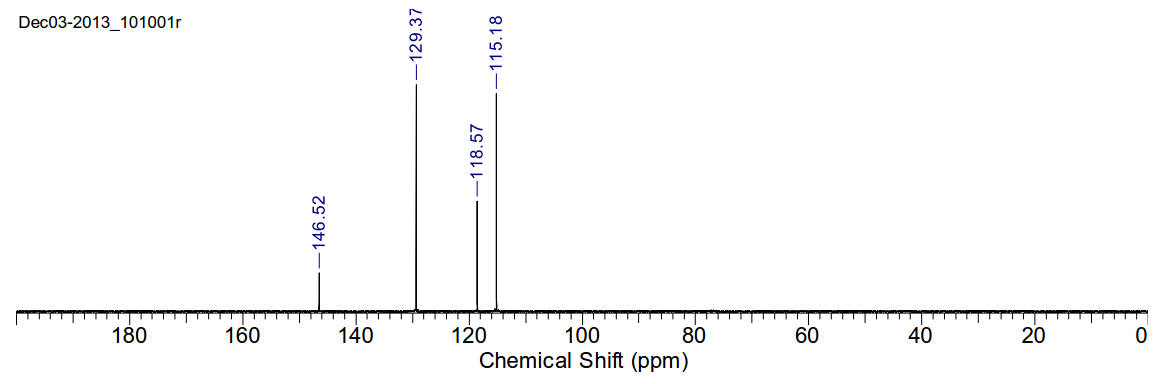
Aniline
C6H7N 93.13gmol-1
A yellow-pink oil miscible with chloroform and very
slightly soluble in water.
1H-NMR (400MHz, CDCl3) δ 7.13 (t,
J=7.78Hz, 2H), 6.74 (t, J=6.34Hz, 1H), 6.64 (d, J=7.92, 2H), 3.58 (s, 2H)
13C-NMR (400MHz, CDCl3) δ 146.52,
129.37, 118.57, 115.18
13C-NMR (400MHz, D2O) δ 146.04,
129.44, 119.48, 116.37
4-chloroaniline
C6H6ClN 127.57gmol-1
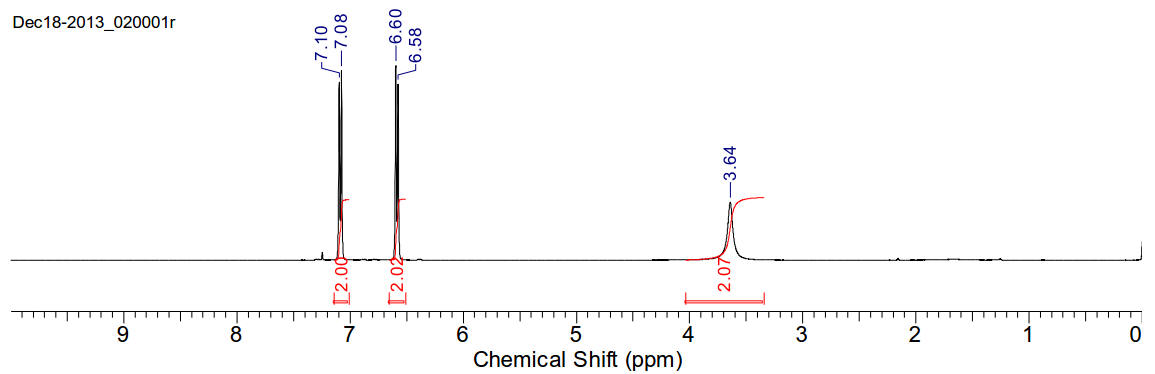
1H-NMR (400MHz, CDCl3) δ 7.09 (d,
J=8.31Hz, 2H), 6.59 (d, J=8.22Hz, 2H), 3.64 (s br, H).
1H-NMR (400MHz, D2O) δ 7.09 (d,
J=8.61Hz, 2H), 6.69 (d, J=8.61Hz, 2H), 4.69 (s br, 2H).
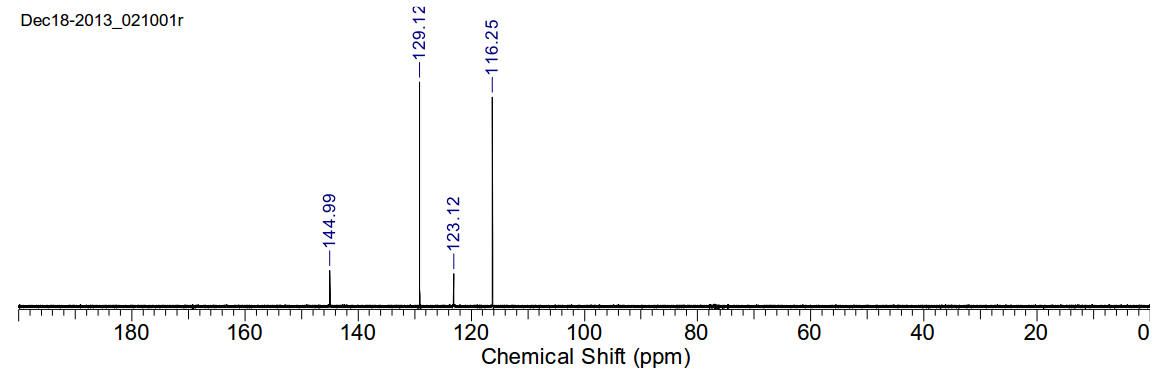
13C-NMR (400MHz, CDCl3) δ 144.99,
129.12, 123.12, 116.25.
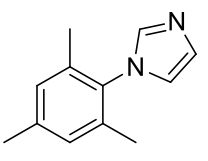
1-(2,4,6-trimethylphenyl)imidazole
C12H14N2 186.25gmol-1
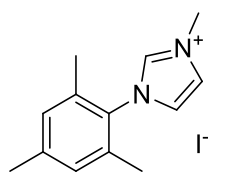
1-methyl-3-(2,4,6-trimethylphenyl)imidazole
C13H17IN2 328.19gmol-1
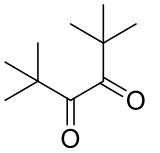
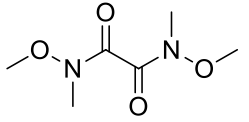
2,2,5,5-tetramethyl-3,4-hexandione
C10H18O2 170.25gmol-1
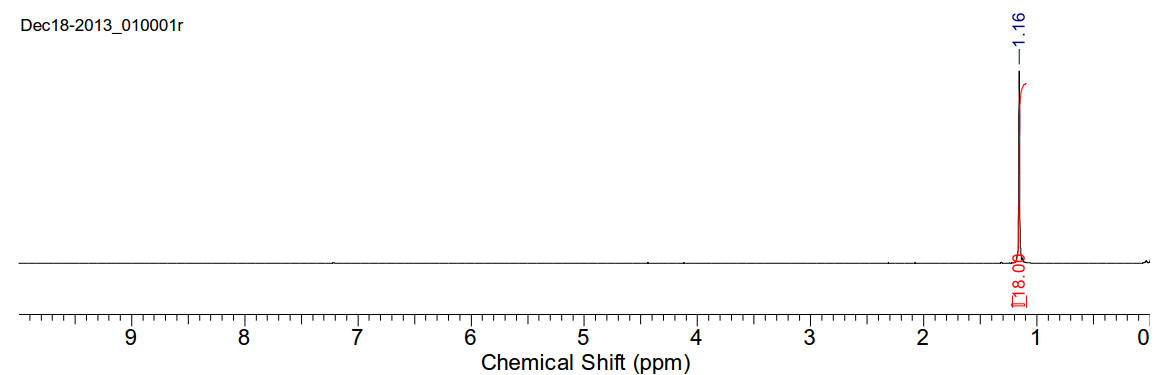
1H-NMR (400MHz, CDCl3) δ 1.16 (s,
18H).
13C-NMR (400MHz, CDCl3) δ 209.40,
40.08, 24.50.
Cyclohexane-1,2-dione
C6H8O2 112.13gmol-1
3,3,6,6-Tetramethyl cyclohexan-1,2-dione
C10H16O2 168.23gmol-1
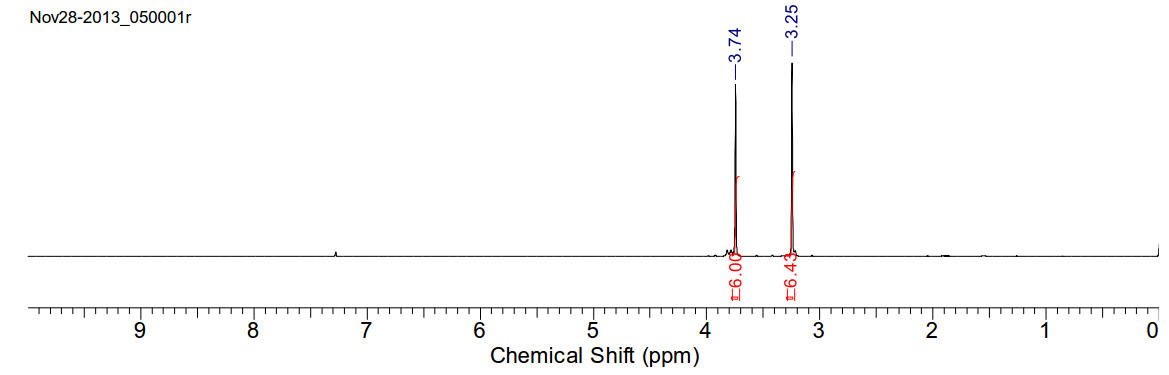
N,N’-dimethyl-N,N’-methoxy oxaldiamide
C6H12N2O4
176.17gmol-1
White needles very soluble in chloroform.
1H-NMR (400MHz, CDCl3) δ 3.25 (s, 6H), 3.74 (s, 6H)
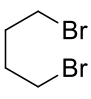
1,4-dibromobutane
C4H8Br2 215.91gmol-1
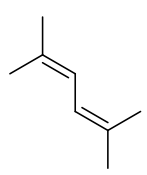
2,6-dimethyl hex-2,4-ene
C8H14 110.20gmol-1
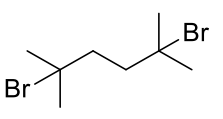
2,6-dimethyl-2,6-dibromohexane
C8H16Br2 272.02gmol-1
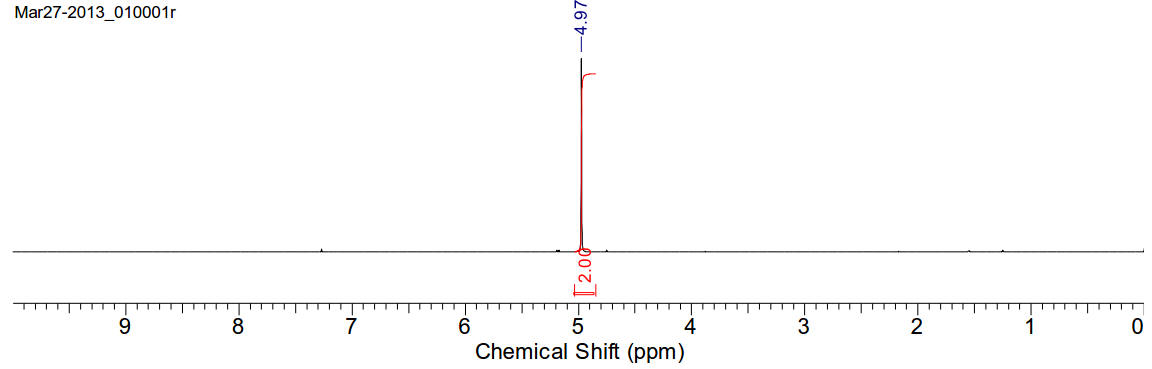
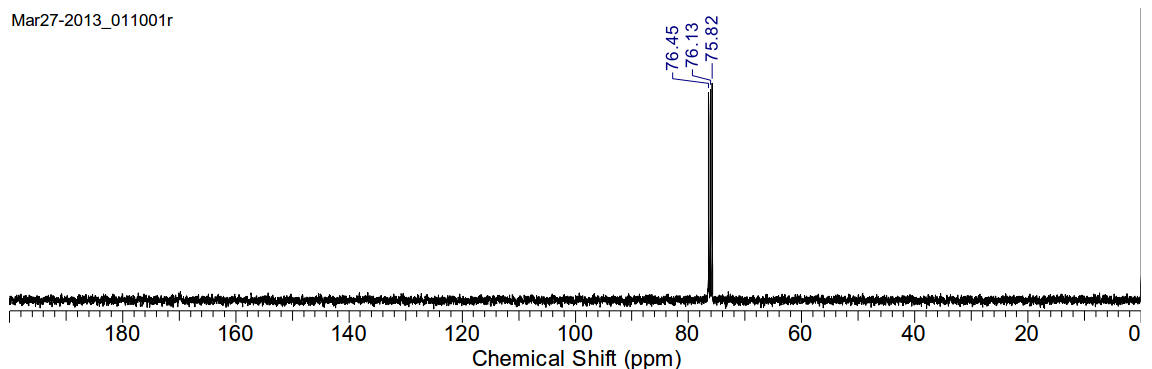
Chloroiodomethane
CH2ClI 176.38gmol-1

1H-NMR (400MHz, CDCl3) δ 4.99 (s, 2H)
13C-NMR (400MHz, CDCl3) 76.13 (t)
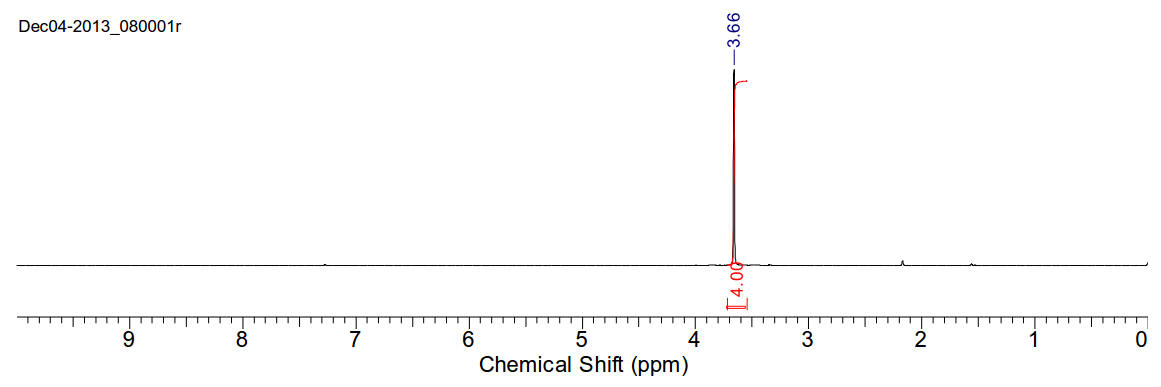
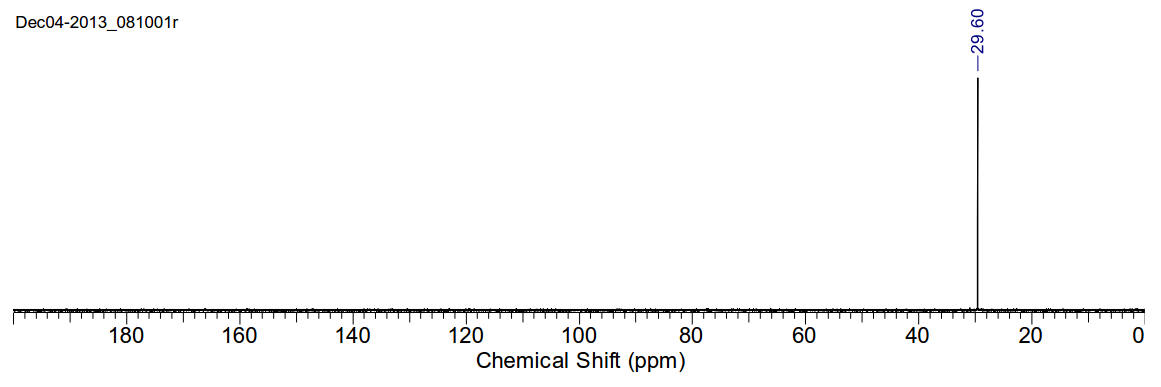
1,2-Dibromoethane
C2H4Br2 187.86gmol-1
1H-NMR (400MHz, CDCl3) δ 3.66 (s, 4H)
13C-NMR (400MHz, CDCl3) δ 29.60
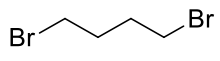
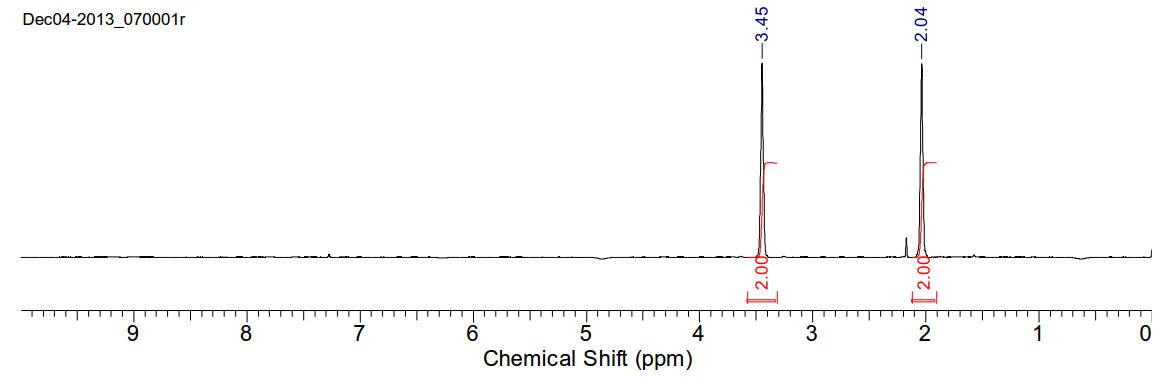
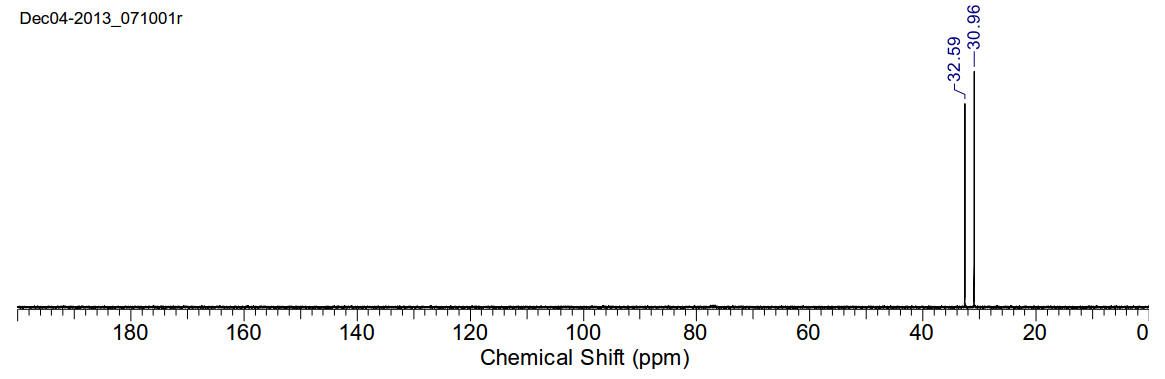
1,4-dibromobutane
C4H8Br2 215.91gmol-1
1H-NMR
(400MHz, CDCl3) δ 3.45 (s, 2H), 2.04 (s, 2H)
13C-NMR
(400MHz, CDCl3) δ 32.59, 30.96
Diiodomethane
CH2I2 267.84gmol-1

1H-NMR (400MHz, CDCl3) δ 3.89 (s, 2H)
Dichloromethane
CH2Cl2 84.93gmol-1

1H-NMR (400MHz, CDCl3) δ 5.33 (s, 2H)
Chloromethyl ethyl ether
A dangerously carcinogenic liquid miscible with chloroform
but decomposes on contact with water.
C3H7OCl 94.54gmol-1
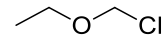
1H-NMR (400MHz, CDCl3) δ 5.52 (s, 2H), 3.76 (q, J=7.08,
2H), 1.27 (t, J=7.09, 3H)
13C-NMR (400MHz, CDCl3) δ 82.99, 66.07, 14.39
1,3-dimesitylimidazolium chloride (IMes·Cl)
C21H25N2Cl
340.89gmol-1
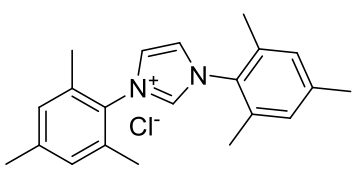
1H-NMR
(249.87Mhz, CDCl3): δ
ppm 2.19 (s, 12H, o-CCH3),
2.34 (s, 6H, p-CCH3),
7.03 (s, 4H, m-H),
7.63 (s, 2H, NCH=CHN),
10.89 (s, 1H, NCHN). 13C-NMR
(249.87Mhz, CDCl3): δ
ppm 17.67 [4C, o-CCH3],
21.18 [2C, p-CCH3],
124.37 [2C, NC=CN],
129.95 [4C, m-C],
130.63 [2C, p-C],
134.09 [4C, o-CCH3],
139.81 [2C, i-CN], 141.36 [2C, NC=CN].
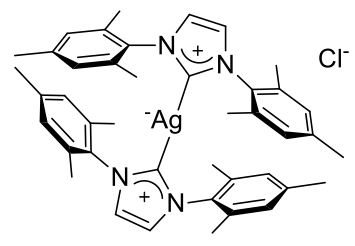
Bis(1,3-bismesityl-imidazol-3-ium-2-yl)argentate chloride
C42H50N2AgCl
754.19gmol-1
A chalky
white solid insoluble in hexane but soluble in dichloromethane.
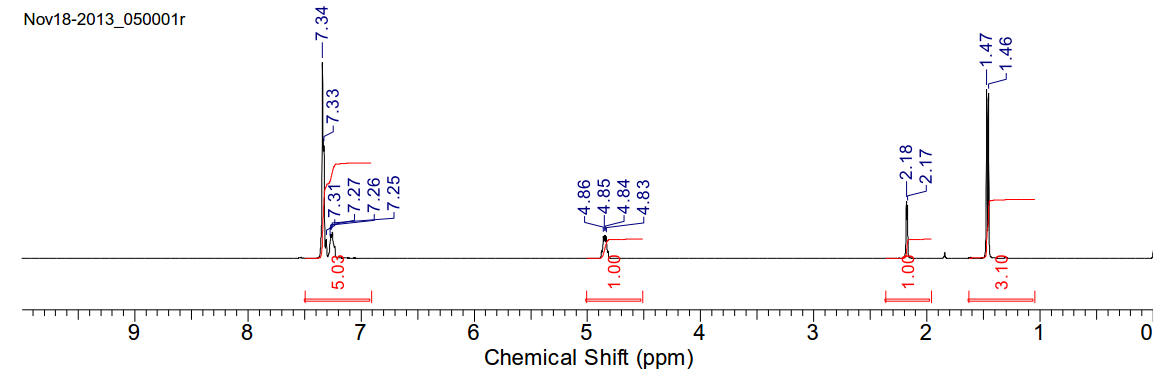
(1s)-1-phenylethanol
Soluble in chloroform.
1H-NMR (400MHz, CDCl3)
δ 1.46 (d, J=6.5Hz, 3H), 2.18 (d, J=3.6Hz, 1H), 4.85 (qd, J=6.5Hz, 3.4Hz,1H),
7.30-7.36 (m, 5H)
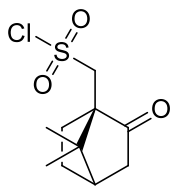
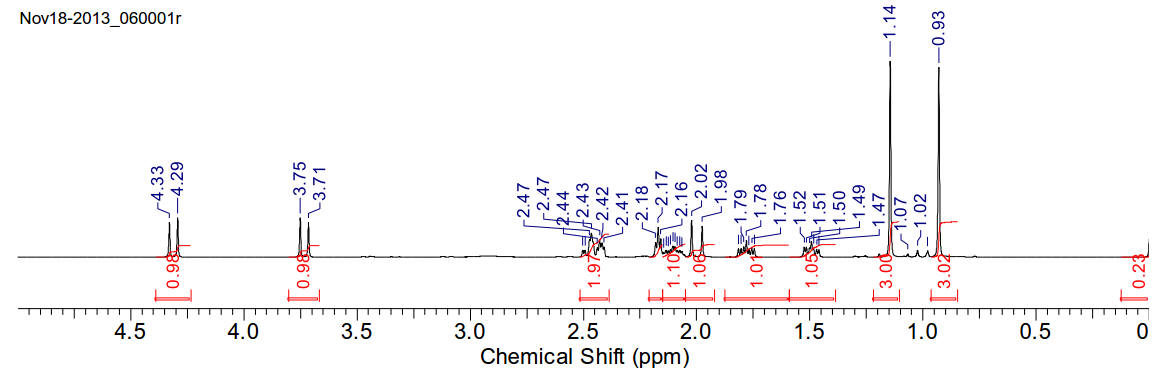
(1R)-(−)-10-Camphorsulfonyl chloride
1H-NMR (400MHz, CDCl3) δ 0.93 (s,
3H), 1.14 (s, 3H), 1.49 (ddd, J=12.72Hz, 9.24Hz, 3.86Hz), 1.78 (ddd, J=13.96Hz,
9.37Hz, 4.65Hz, 3H), 2.00 (d, J=18.68Hz, 1H), 2.11 (m, J=16.18Hz, 8.13Hz,
3.94Hz, 3.94Hz, 1H), 2.17 (t, J=4.55Hz, 1H), 2.45 (m, 2H), 3.73 (d, J=14.57Hz,
1H), 4.31 (d, J=14.67Hz, 1H)
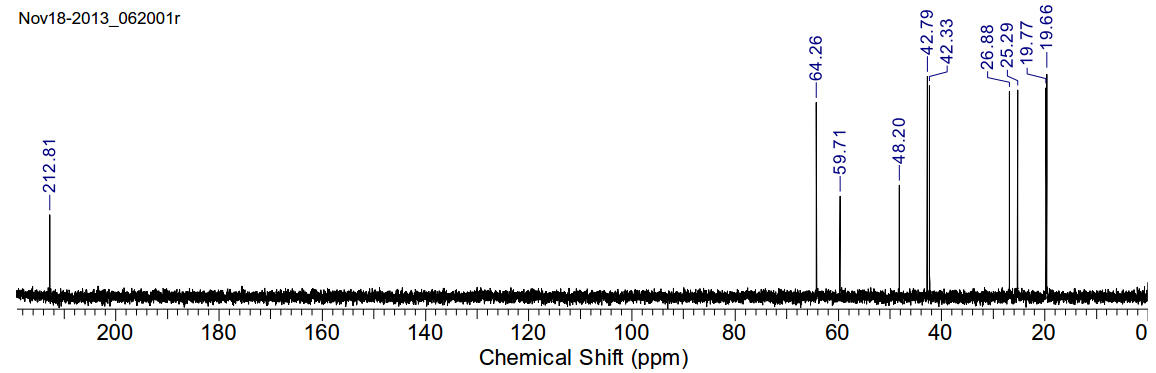
13C-NMR (400MHz, CDCl3) δ 19.66,
19.77, 25.29, 26.88, 42.33, 42.79, 48.20, 59.71, 64.26, 212.81
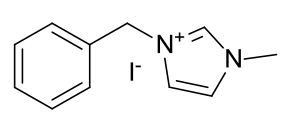
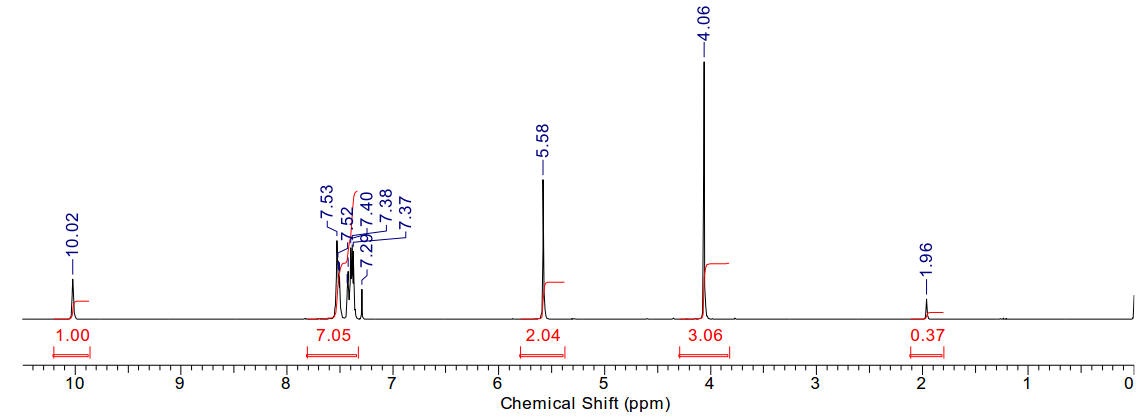
1-methyl-3-benzyl imidazolium iodide
|
|
A light
yellow crystalline solid |
Solid is purified by trituration with 10% ethanol in ether.
Material is reasonably soluble in spectroscopic grade CDCl3 at RTP
and very soluble in DMSO. In chloroform, the methyl protons (4.05ppm, 3H), the
benzylic protons (5.58ppm, 2H) and the imidazolium C2-proton
(9.98ppm, 1H) are identifiable as clear resonants. The imidazolium-4,5 protons
(2H) are clustered in the phenyl aromatic region (5H) along with CHCl3.
Found 13H.
In DMSO, similarly distinct resonants are observed but far
greater resolution is observed of the aromatic region. The phenyl proton
resonants overlap in the form of a typical aromatic multiplet, but the
imidazole-4,5 proton resontants (7.73ppm, 1H and 7.81ppm, 1H) are distinct.
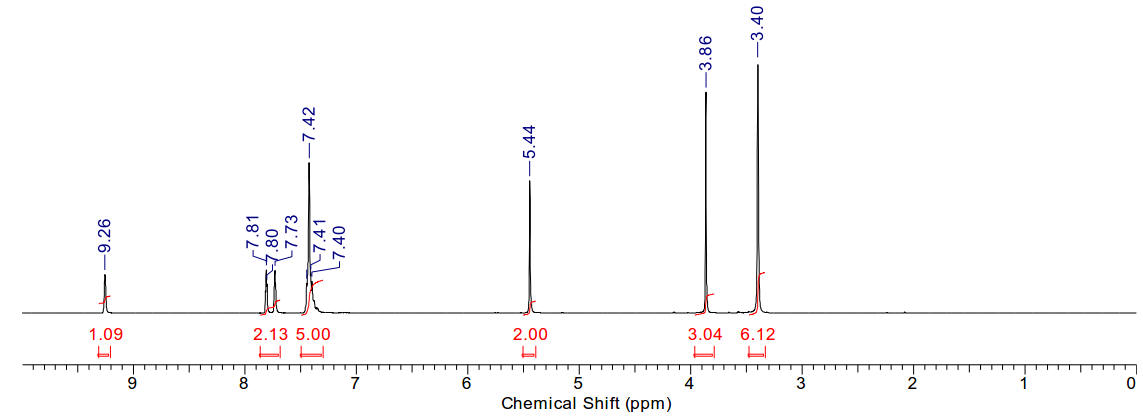
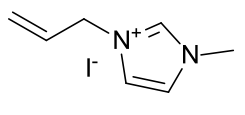
1-allyl-3-methyl imidazolium iodide
|
|
A light
yellow crystalline solid |
Material is soluble in spectroscopic grade CDCl3
at RTP and very soluble in DMSO. In chloroform, the methyl protons (4.14ppm,
3H), the allylic protons (5.04ppm, d, 2H), the imidazolium C2-proton
(9.81ppm, 1H) and the imidazolium-4,5 protons (7.72 and 7.59, 2H) are
identifiable as clear resonants. The terminal olefinic protons resonate at
between 5.59 and 5.47ppm, m). The allyl methine resonance with a distinct
multiplet (6.14-6.01ppm, m, 1H). Found 11H.
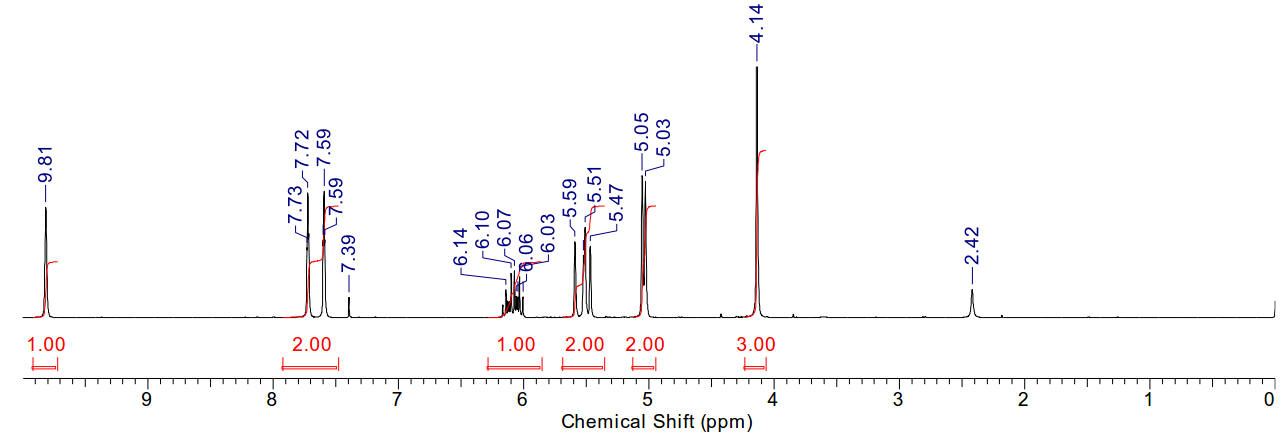
In DMSO, the imidazolium-4,5 protons are not distinct from
one-another.
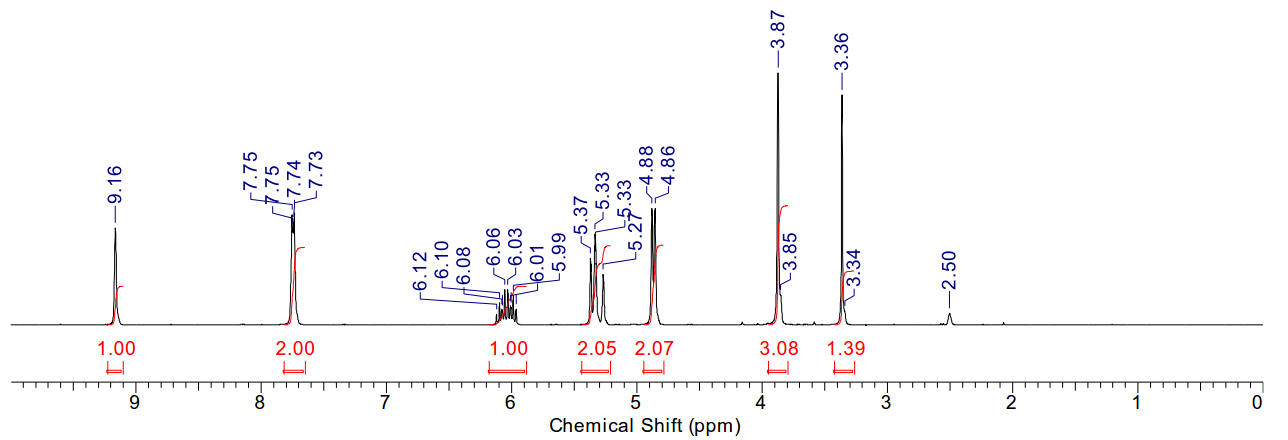
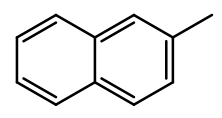
2-methylnaphthalene
|
|
Clear
crystalline plates mp 34 –
36ºC |
Material is soluble in spectroscopic grade CDCl3
at RTP and very soluble in DMSO. In chloroform, the methyl protons are distinct
(2.48ppm, 3H). The aromatic region is typical.
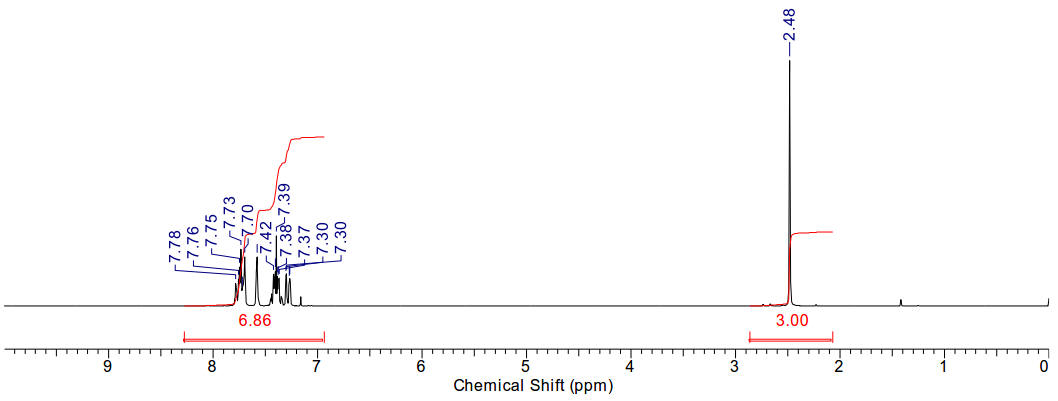
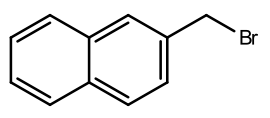
2-bromomethylnaphthalene
|
|
A tan
powder mp 49 –
51ºC |
Material is soluble in spectroscopic grade CDCl3
at RTP and very soluble in DMSO. In chloroform, the bromomethyl protons are
distinct (4.66ppm, 2H). The aromatic region is typical. A conversion ratio of
90% is ascertained by comparison of the product 2-bromomethyl resonant integral
and the starting material methyl resonant integral.
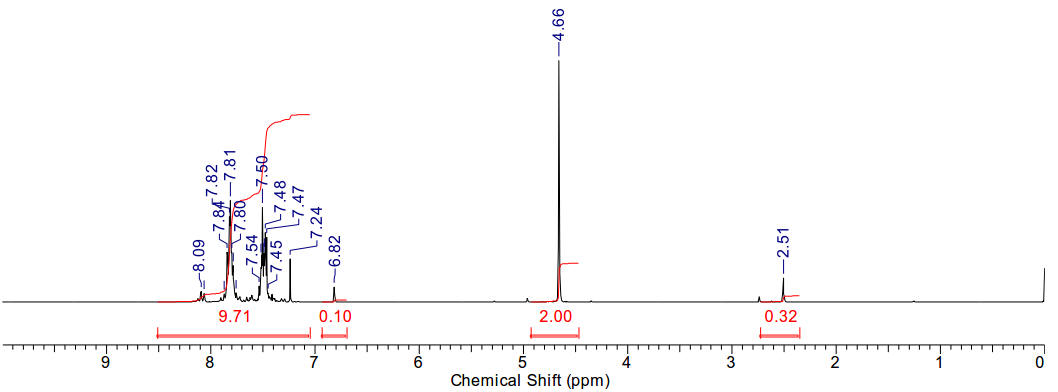
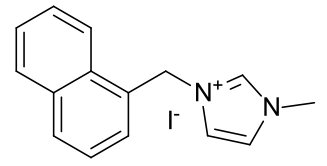
1-naphthyl-3-methyl imidazolium iodide
|
|
A light
yellow crystalline solid |
Material is soluble in spectroscopic grade CDCl3
at RTP and very soluble in DMSO. In chloroform, the methyl protons (4.14ppm,
3H), the allylic protons (5.04ppm, d, 2H), the imidazolium C2-proton
(9.81ppm, 1H) and the imidazolium-4,5 protons (7.72 and 7.59, 2H) are
identifiable as clear resonants. The terminal olefinic protons resonance at
between 5.59 and 5.47ppm, m). The allyl methine resonance with a distinct
multiplet (6.14-6.01ppm, m, 1H). Found 11H.
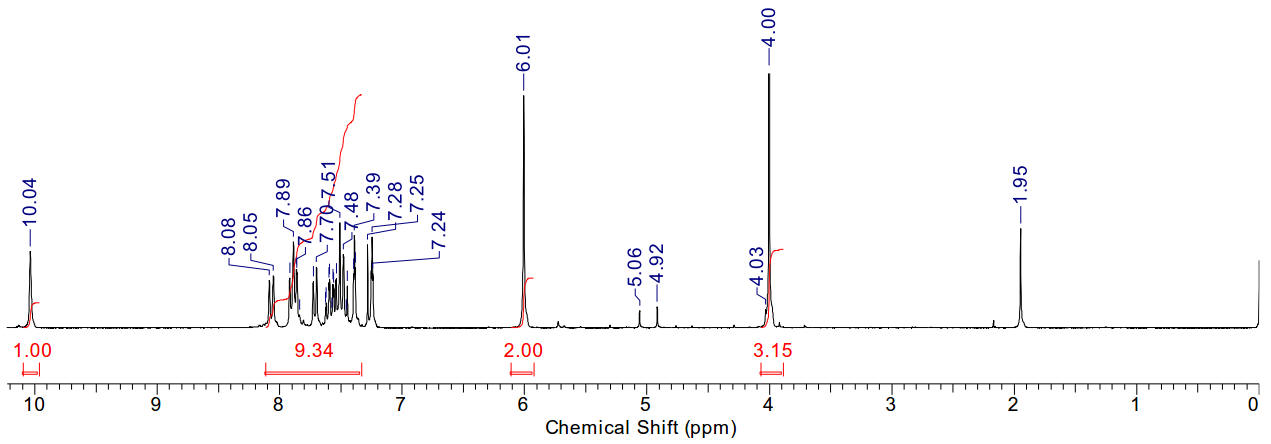
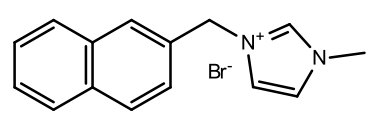
2-naphthyl-3-methyl imidazolium iodide
|
|
A brown
oil |
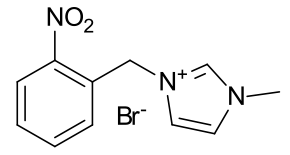
1-(2-nitrobenzyl)-3-methyl imidazolium iodide
|
|
A clear
crystalline solid |
Material is soluble in spectroscopic grade CDCl3
at RTP and very soluble in DMSO.
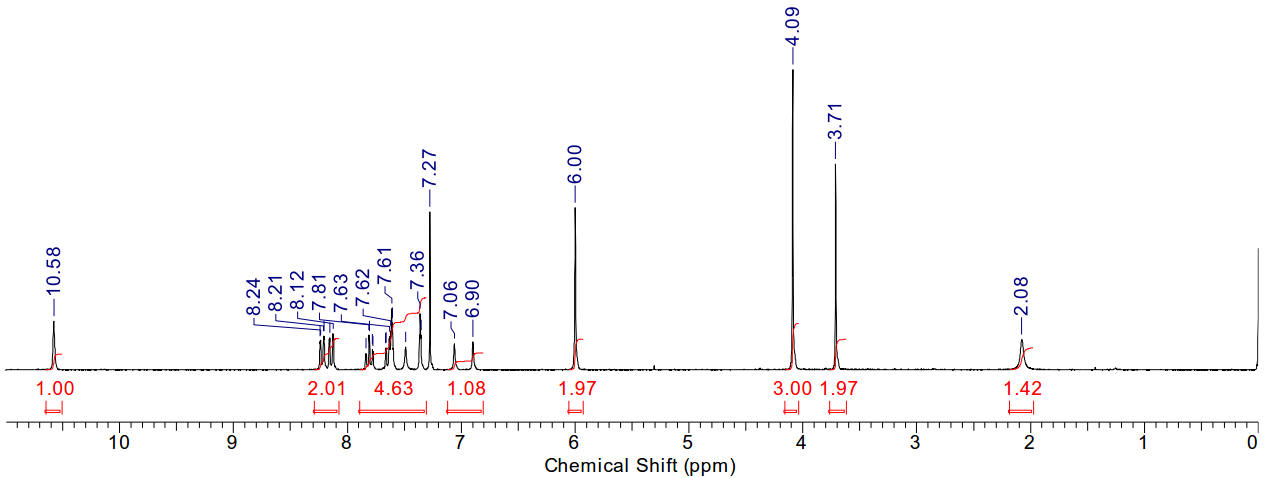
The peak at 3.71 represents an impurity of methylimidazole
(it is congruent with the peaks at 7.06 and 6.90)
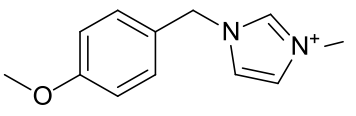
1-(para-methoxybenzyl)-3-methyl
imidazolium bromide
|
|
Brown oil |
May11-2009-LMH_250\001r
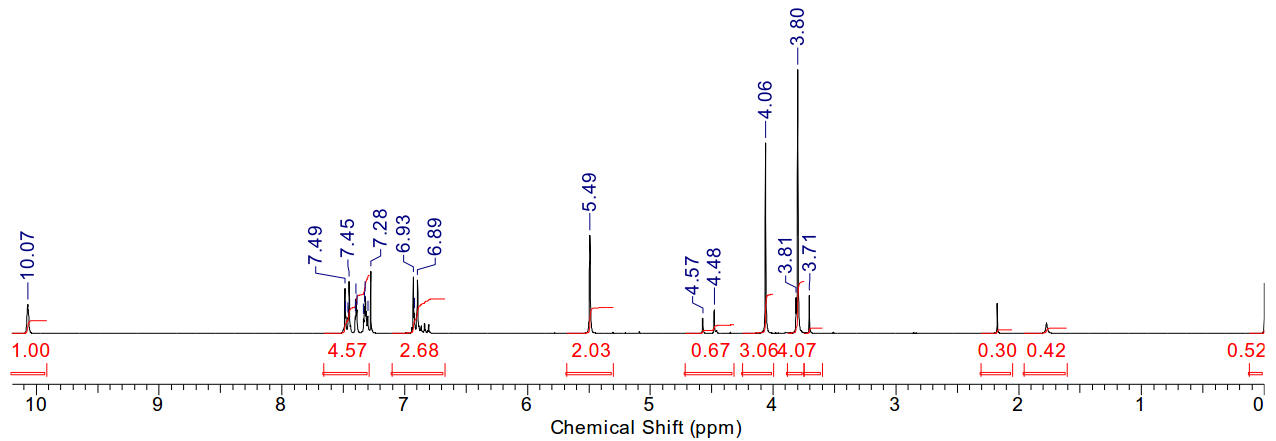
Material is soluble in spectroscopic grade CDCl3
at RTP and very soluble in DMSO. In chloroform, the 3-methyl protons are present
at a position typical for these compounds (4.06ppm, s, 3H), as are the methoxy
protons typical of the PMB group (3.80ppm, s, 3H). The benzylic protons
(5.49ppm, s, 2H) and the imidazolium C2 proton (10.07ppm, s, 1H) are clearly
visible.