Isolation of Imidazolium Salts by Reversed-Phase Flash Chromatography
Chromatography separation techniques are enabled by the partition phenomena of analytes between a stationary phase and a mobile phase of differing adsorption characteristics. Reversed phase chromatography is so named because the stationary phase polarity is ‘reversed’ in relation to the historically employed silica and alumina stationary phases. These reversed stationary phases typically comprise of a normal polar stationary phase matrix that has been derivatized with a non-polar ligand.
The retention phenomenon is determined by the interaction between the analyte and the molecular surface to which it can access, and thus can be modified by introducing new surface functionalities to the matrix. Silica gel is the base matrix of many reversed stationary phases due its cost, abundance, the relative ease with which surface is derivatised and the wide range of ligands that can be successfully immobilized. Common ligand surface functionalities introduced include the simple straight chain alkyls, octyl and octadecyl, but also more complex ligands such as calixarenes and cyclodextrins.
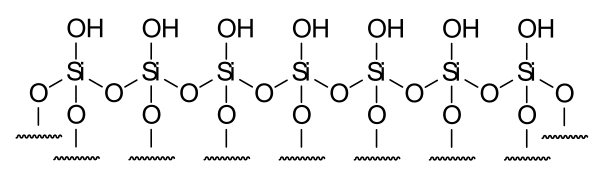
The highly polar surface of non-derivatized silica
The retention of an analyte by a reversed phase matrix is dependent upon the specific nature of the surface functionalities. Selection of the appropriately derivatized silica is essential for good separations and the choice is significantly influenced by the analyte polarities. Typically, the more hydrophobic analytes adhere strongly to octadecyl derivatized silica and so the shorter chain, less hydrophobic octyl derivatives are preferred. More polar analytes achieve better separation with strongly hydrophobic stationary phases and thus imidazolium salts may be well separated with octadecyl derivatized silica.
This octadecyl derivatized silica gel was prepared by the established literature procedure: Silica gel 60 (230 – 400mesh) suspended in toluene was treated with octadecyltrichlorosilane, thoroughly washed and dried.
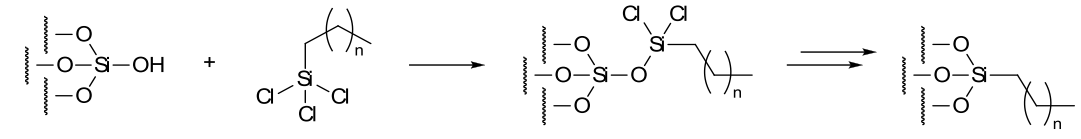
Schematic for the surface derivatisation of silica
Since the derivatising chains are long, the derivatization process does not go to completion. What results is a silica matrix with randomly un-derivatized surface sites. These free silol groups interact with the analytes and thus the separations are not always reproducible. This problem is at least in part overcome by end-capping the freshly derivatized silica, but to achieve reproducibility the end-capping procedure must be reliable. Typical end-capping reagents are smaller derivatization ligands bearing methyl substituents such as trimethylchlorosilane or the now preferred hexamethyldisilizane which decrease free surface silol groups significantly. The effect of the remaining free silol groups remained considerable, as strong interactions with polar analytes causes streaking. The addition of an ion-pairing agent reduces the affinity of these analytes for free silol groups by locally associating with the polar and ionic functionalities.
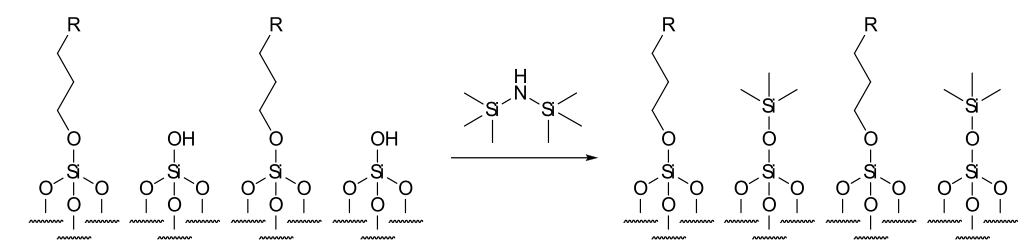
Figure: End-capping silica with HMDS
In contrast to normal phase chromatography, the mobile phases employed are significantly polar and commonly aqueous. A less polar organic modifier is added to lower the polarity of the mobile phase, which in turn increases the eluting strength. Typical modifiers include methanol and higher alcohols, THF and acetonitrile. Ion-pairing agents can be introduced to the solvent system to adjust the analyte hydrophilicities. Trifluoroacetic acid [ ] is one such agent that pairs with hydrophilic analytes, locally enhancing affinity for the reverse phase matrix and reducing affinity for surface free silol groups. Triethylamine can be used in other situations to similar effect.
As salts, imidazolium compounds are very polar. With normal phase chromatography (Silica, 5% methanol in dichloromethane) the imidazolium salts elute long after the bulk of impurities (Rf=0.6 and 0.95 respectively) but suffer from contamination with streaking impurity. By applying a reversed phase protocol, the product being eluted first avoids contamination. The ideal goal of solvent system selection is to find one that moves the imidazolium salt with the solvent front but does not move the impurities from the base line. Realistically, an Rf value of 0.4 for the imidazolium salt is sought with the closest eluting impurities being no less than 0.2 lower.
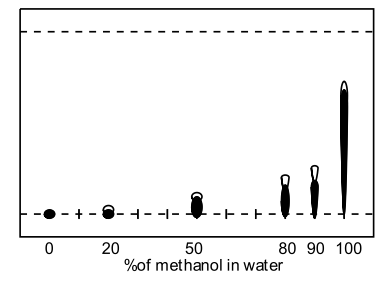
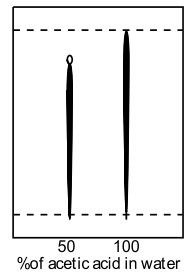
Basing the mobile phase on water in which the impurities are insoluble and the product is only sparingly soluble, a range of water-miscible solvents were assayed to find an appropriate modifier to permit efficient elution of the product and less or negligible elution of impurities. The TLC was formed with C18 derivatized silica on glass (F254) and the product visualized using UV-254nm as a blue-fluorescent spot; whilst the impurities were easily visible as a black streak. No appreciable elution was observed with neat water, with or without ion-pairing agents. Substitution of water with brine resulted in no beneficial change. Extensive streaking occurred without the inclusion of acidic ion-pairing agent (trifluoroacetic acid, acetic acid, formic acid) but basic agents (triethylamine) did not improve the resolution.
Figure: TLC of IMes·HCl, Silica C18 on glass, 90% methanol in water, UV-254nm without ion-pairing agent (left), with formic acid (middle) and TFA (right)
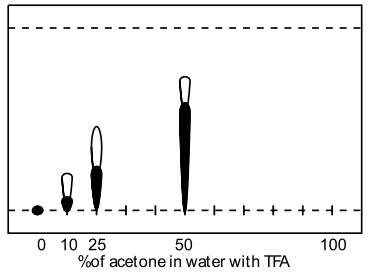
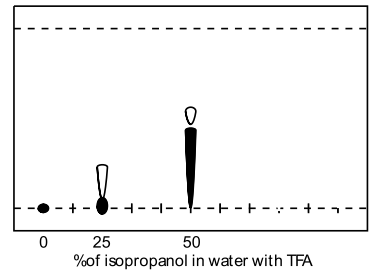
A range of modifiers was examined (methanol, acetonitrile, THF, acetone and isopropanol) including several tri-component solvent systems. Very high water contents significantly retarded elution, and the presence of acidic ion-pairing agent had dramatic effect on the obtained retention factors, ie., comparison of 50% aq. methanol (Rf=0.1) to 50% aq. methanol with formic acid (Rf=0.55). TFA was found to give superior spot formation and resolution.
 |
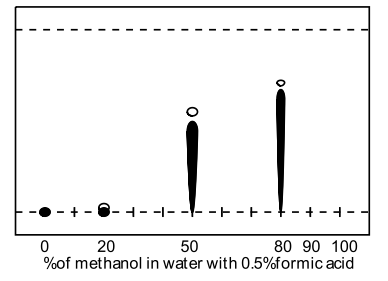 |
| C18 TLC Methanol in water | C18 TLC Methanol in water with formic acid |
| Nb: The hollow spot represents imidazolium salt and the solid spot represents impurities. | |
 |
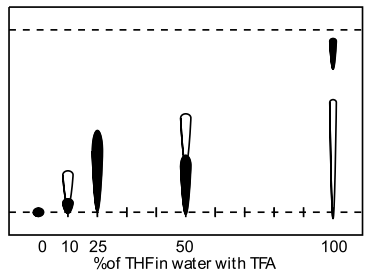 |
| Acetic acid in water | THF in water with TFA |
 |
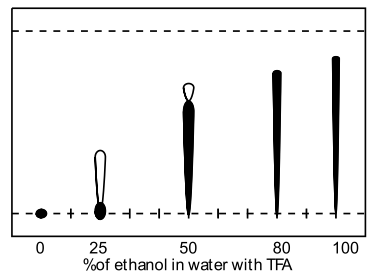 |
| Acetone in water with TFA | Ethanol in water with TFA |
 |
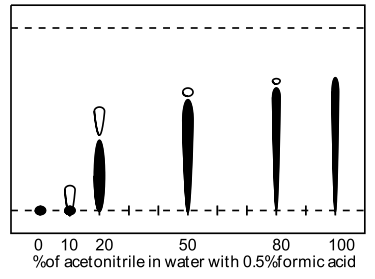 |
| Isopropanol in water with TFA | Acetonitrile in water with formic acid |
An unusual order of elution was found for neat THF with 1% TFA as the imidazolium salt eluted last (Figure 8, lane 5). The impurity separated with good resolution and whilst this is not a typical mobile phase for reversed-phase chromatography it may be transferable to the flash column.
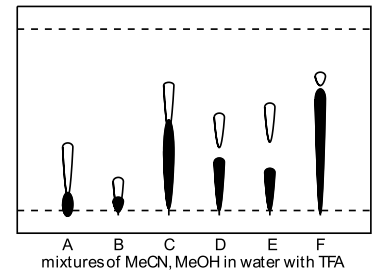 |
A: 20% MeCN + 10% MeOH B: 10% MeCN + 20% MeOH C: 30% MeCN + 10% MeOH D: 20% MeCN + 20% MeOH E: 10% MeCN + 30% MeOH F: 50% MeCN + 25% MeOH composition in water with 1% TFA |
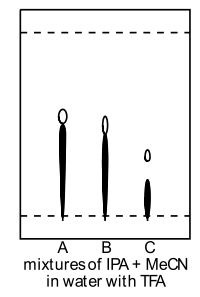 |
A: 30% IPA + 10% MeCN B: 20% IPA + 20% MeCN C: 10% IPA + 30% MeCN composition in water with 1% TFA |
As can be seen, none of the solvent systems examined gave excellent resolution, but of the solvent systems assayed, 10% acetonitrile and 30% methanol with TFA in water (Figure 13, Lane E) was found to be the best. However, when this solvent system was transferred to a reversed phase flash column, the elution rate was prohibitively slow and excessive use of solvent was required. Despite this the reversed phase flash chromatography was successful in achieving separation of the pure imidazolium salt (C18-reversed phase flash chromatography, 10% Acetonitrile + 30% methanol + 1% TFA in water).
Avoiding the time and excessive use of solvent of the flash column, a more convenient mode of isolation was found to be reversed-phase dry-flash chromatography through a small plug of C18 reversed phase silica. The eluent solvent system of 10% aqueous acetonitrile with 0.5% TFA was found to be effective for this dry-flash chromatography. A sintered-funnel was charged with a slurry of C18 reversed phase silica (25g) in the eluent solvent mixture with the vacuum applied to give a silica plug of one inch height. This was evenly pressed flat with a glass sample vial or glass-rod. The plug was pre-washed with 100mL of the eluent and dried briefly with the vacuum aspirator. The sample was dry loaded in an even layer adsorbed onto minimal reverse phase silica gel and a quantity of washed low iron sand applied. A small filter paper was placed on top of the sand and another layer of sand applied. The collections were made isocratically with 40mL aliquots of eluent, sucking the silica dry between each fraction.
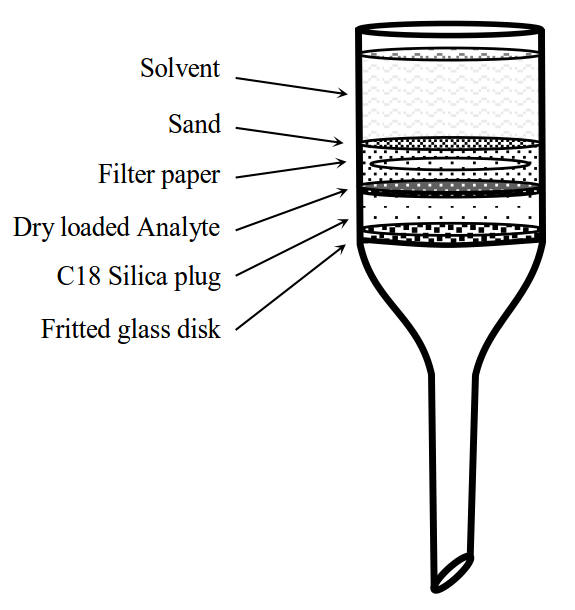
Figure: Construction of the dry-flash column
References
Lochmuller, C.; Marshall, D., Analytica Chim. Acta., 1982, 142, 63-72
Petritis, K.N.; Chaimbault, P.; Elfakir, C.; Dreux, M.; J. Chromatogr.A, 1999, 833, 147.
Harwood, L. M., Aldrichimica Acta., 1985, 18, 25.