ORCA - Electronic and Spectroscopic Properties of Olefins
Although restricted in which excited energy levels are accessed during the computation, to get a rough idea of the UV-Vis spectrum it is possible to invoke ORCA with an *.inp file in which the default settings can be sufficient. The particular settings contained within the ORCA *.inp file employed to obtain the spectra presented here are as follows:
! ZINDO/S
! PrintBasis
%cis
nroots 8 # the number of excited states to be calculated.
maxdim 30 # the maximum dimension of the expansion space in the Davidson procedure.
# Triplets true : do triplets states
# EWin -3,100 (orbital energy window in Eh)
end #cis
%output
print[p_mos] 1
end #output
Once the geometry of the molecule has been optimised, many molecular modelling graphical user interfaces offer a functionality to produce ORCA input files. Despite the settings being different, the generated ORCA files can be viewed and the .xyz section obtained, which contains the atomic coordinates, spin multiplicity and charge. A new .inp file can be created using the code above followed by the section beginning * xyz. See example input file for (s-trans)-buta-1,3-diene.
Effects of substitution on the UV-Vis spectra of olefins
| (s-trans)-buta-1,3-diene | ||
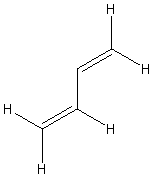 |
231 | 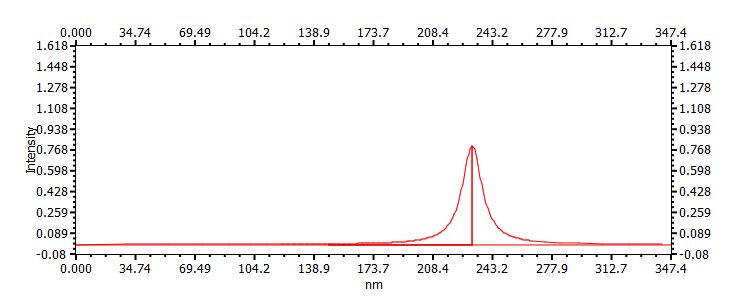 |
| (s-trans,E)-1-chlorobuta-1,3-diene | ||
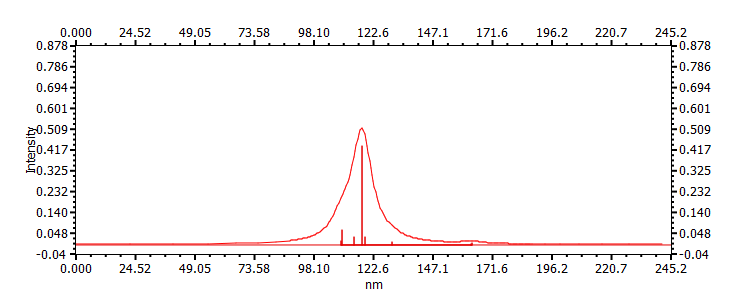
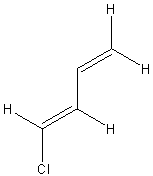 |
117, 109, 115, |
 |
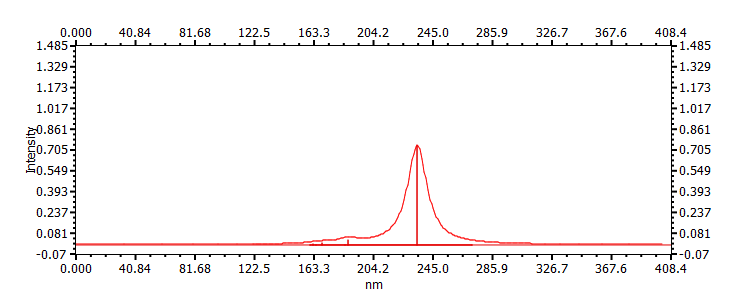
| (s-trans,Z)-1-chlorobuta-1,3-diene | ||
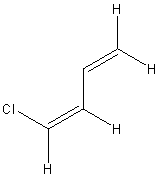 |
233, 186, 164 |
 |
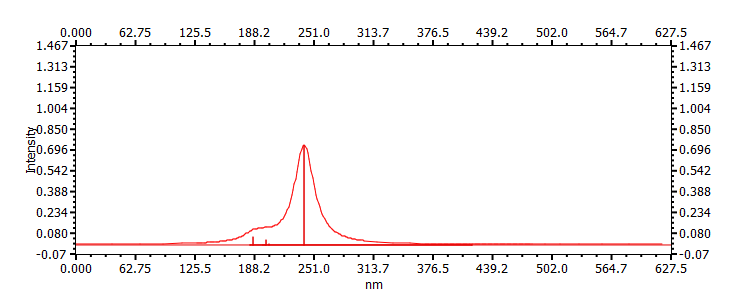
| (s-trans)-1,1-dichlorobuta-1,3-diene | ||
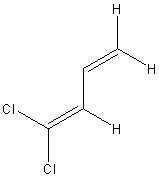 |
239, 186, 199 |
 |
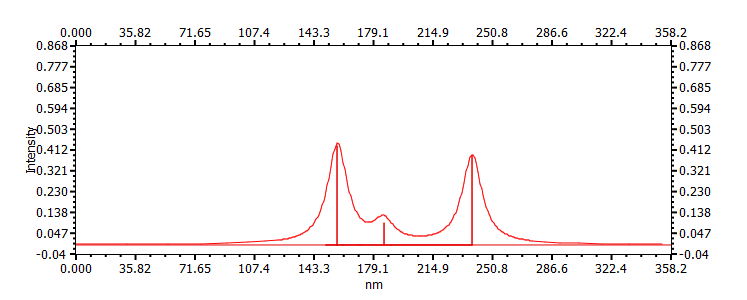
| (s-cis)-buta-1,3-diene | ||
 |
 |
|
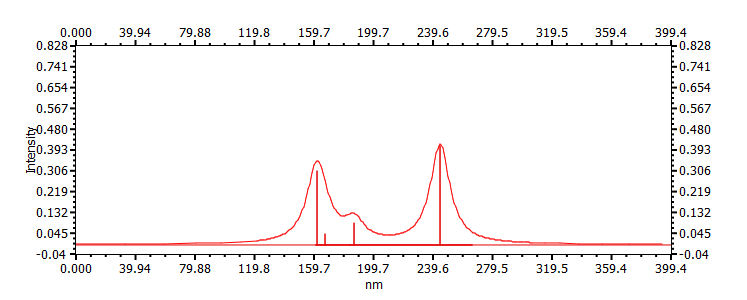
| (s-cis,E)-1-chlorobuta-1,3-diene | ||
 |
244, 160, 187 167 |
 |
| (s-cis,Z)-1-chlorobuta-1,3-diene | ||
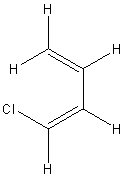 |
||
| (s-cis)-1,1-dichlorobuta-1,3-diene | ||
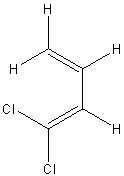 |
249, 370, 411, 400, |
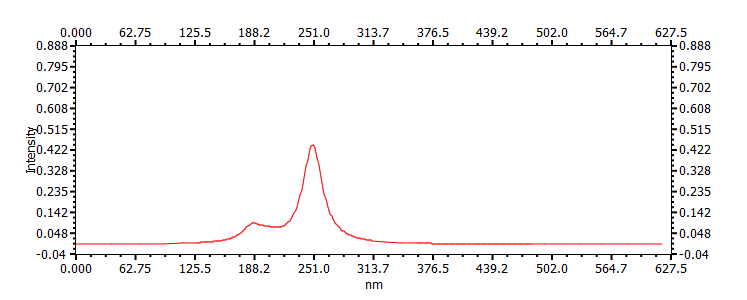 |
| 241 | 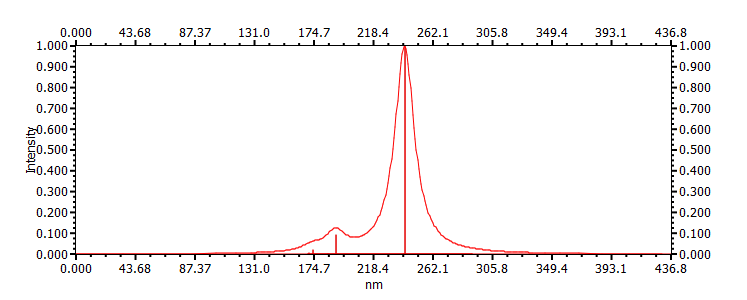 |
|