The current state of melatonin receptor exploration and exploitation: recent and topical advances
 |
The Design of Melatonergic AgonistsPart 4Summary and Prospectsby Phil |
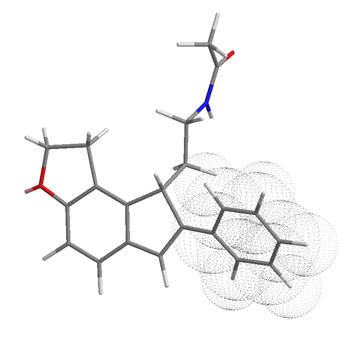
Summary of features key to dynamics
Over the past twenty years a wealth of knowledge has been acquired concerning the requirements of melatonergic activity. Key structural features and parameters of the ligand have been ascertained for good drug like activity. Also elucidated indirectly is information regarding the complementary receptor.
 |
|
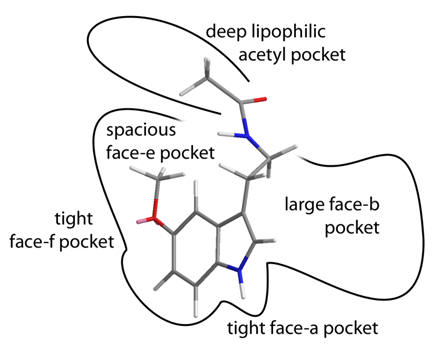
Figure 33 | Summary of the receptor sub-pockets
The activity of the two currently available melatonergic drugs can be easy rationalised with this working model of the receptor.
 |
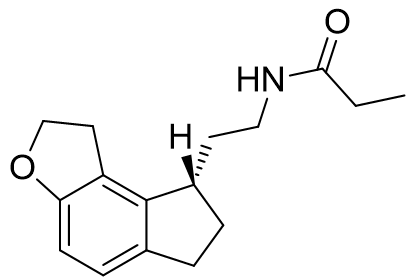 |
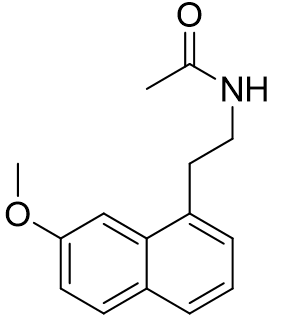
Figure 34 | Agomelatine and Ramelteon revisited
Agomelatine is simply the naphthalene bioisostere of indole. This compound is more lipophilic than melatonin and has an appreciably longer half-life. Ramelteon exploits several optimisations: the acetyl group is extended into the lipophilic pocket, the C5-O alkyl group is restrained in its active conformation to the face-e pocket, saturation and substitution of the pyrrolic component results in the ethylamido side-chain subtending below the plane. Ramelteon is highly selective and also incredibly potent. Agomelatine on the other hand is far less receptor sub-type selective. In fact, so lax is the selectivity that is also has antagonist activity at the 5-HT2C receptor - this side-effect is actually very fortuitous. Over stimulation of the 5-HT2C receptor is the cause of anxiety prevalent during the early phase of treatment with SSRI's. Hence Agomelatine is particularly useful as an SSRI adjunctive agent to offset symptoms of anxiety until receptor desensitisation occurs.
Summary of features tolerated pertaining to kinetic optimisation
It is apparent that the pyrrole group is not involved in H-bonding. Whilst the pyrrole binding pocket is not particularly large, it is tolerant of a wide array of diverse functionality. This provides many opportunities for tailoring the kinetic profile. Indeed all current commercial melatonergic drugs exploit this tolerance to extend the elimination half-life. Homologation of the acetyl group is also a convenient way to enhance lipophilicity and thus drug kinetics.
Future Prospects
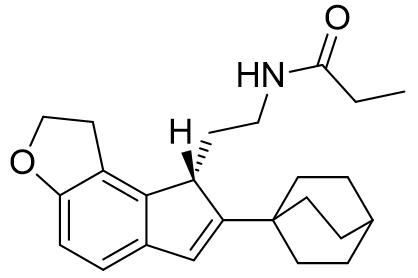
With this model the intelligent design of melatonergic agent de novo is quite conceivable. For example, the C2 binding pocket is yet to be exploited in commercial agonists. The chiral C3 position is a convenient way of displacing the ethylamido chain, but it is also possible to further promote active conformational attainment. The introduction of an additional cycle may operate synergistically with the C3 chirality.
 |
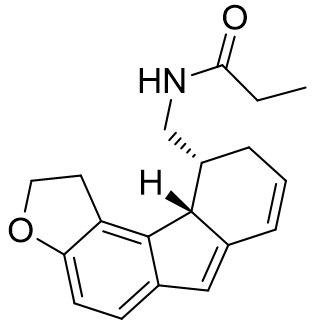 |
Figure 35 | Melatonergic drugs of the future?
Previous: Part 3